£Ø2012?²żĘ½ĒųŅ»Ä££©øł¾ŻŹµŃéÄŚČŻ»Ų“šĪŹĢā£®
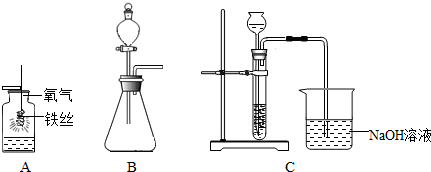
£Ø1£©Ķ¼AĢśĖæŌŚŃõĘųÖŠ¾ēĮŅČ¼ÉÕ£¬
»šŠĒĖÄÉä
»šŠĒĖÄÉä
£¬·Å³ö“óĮæµÄČČ£¬ÓŠŗŚÉ«¹ĢĢåÉś³É£®øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£®
£Ø2£©Ķ¼B×°ÖĆÓĆÓŚÖĘČ”ŃõĘų£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£¬³żĶ¼ÖŠĖłŹ¾µÄ·½·ØĶā£¬ŌŚŹµŃéŹŅÖŠŃõĘų»¹æÉÓĆ
£ØÓĆ»Æѧ·“Ó¦·½³ĢŹ½±ķŹ¾£©·½·ØÖĘČ”£®
£Ø3£©ÓĆĻ”ŃĪĖįŗĶ“óĄķŹÆŌŚC×°ÖĆÖŠ·“Ó¦ÖĘCO
2£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
CaCO3+2HCl=CaCl2+H2O+CO2ӟ
CaCO3+2HCl=CaCl2+H2O+CO2ӟ
£»²¢ŃéÖ¤CO
2ÓŠ¹ŲŠŌÖŹ£¬¹Ū²ģµ½ÉÕ±ÖŠµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£¬µ«ĪŽĘäĖūĆ÷ĻŌĻÖĻó£®ČōÖ¤Ć÷CO
2ÓėNaOHČÜŅŗ·¢ÉśĮĖ»Æѧ·“Ó¦£¬ŅŌĻĀ·½°øŗĻĄķµÄŹĒ
¢Ś¢Ū
¢Ś¢Ū
£ØĢīŠņŗÅ£©£®
¢ŁČ”ÉŁĮæÉÕ±ÖŠŅŗĢåµĪ¼ÓĪŽÉ«·ÓĢŖ
¢ŚČ”ÉŁĮæÉÕ±ÖŠŅŗĢåµĪ¼Ó×ćĮæĻ”ŃĪĖį
¢ŪȔɣĮæÉÕ±ÖŠŅŗĢåµĪ¼Ó×ćĮæŹÆ»ŅĖ®£®




