
��ѧ��������ϵ���У������д����л�ѧ������������������龰����ѧ�Ļ�ѧ֪ʶ�������ش��й����⣮
��1��ˮ���ˮ�����Ƿ������� �����������ѧ�����仯��
��2���ӷ��ӵĽǶȽ��ͣ�ǽ�ڿ���ǽ�����ԭ������ ����
��3����Ӳˮϴ���·������˷ѷ���Ҳϴ�����·����ɰ�Ӳˮ��������ˮ�ķ�������
��4��������Դ�������������з�ֹ����Ʒ��ʴ�������������� ��������ţ����ٰѲ˵������ڳ�ʪ�ĵط����ڸ����г�֧��������۸����г�����Ϳ��
��5��ȫ�桢����������Ĵ�ʳ������ȡӪ�����ʣ������彡���Ļ�����֤����������Ҫ������Ӫ�������� ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����йػ�ѧ����������������
| A����ǧ����ǰ������һ����������ij��ԭ�ӿ�������������� |
| B��������ʵ����������˲�ͬʱ��Ӧ����ԭ���ظ�ʵ�� |
| C���õ������ϵĹ����У�����ԭ�ӱ��ֳ��˸�С���� |
| D����ѧ�����о����ʵ����ʡ���ɡ��ṹ�ͱ仯�����о��仯�����а���������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ij��ѧ��Ӧ����ʾ��ͼ(��ʾ��ԭ�ӣ���ʾ��ԭ��)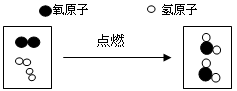
��1���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________��
��2�����ϱ仯�Ļ�����Ӧ������_______________________��
��3�������ͼ����ԭ�ӡ����ӵĽǶȷ������ڻ�ѧ��Ӧ�����У�_______�ɷ֣���________�����ٷ֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��3�֣���ͼ�������������ڵ�ȼ�����·�����Ӧ����ģ��ͼ������ݴ�ͼ�ش��������⣺
��1����Bͼ�н��������ͼ�β���������
(2�����ʷ���Ƕ��������������ڴ������е� ��
��3���˱仯ǰ��û�з����ı����������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����8������Ϊ���г������ʣ����ɱ���Ԫ����ɡ�
| Ԫ������ | �� | ̼ | �� | �� | �� | �� | ͭ |
| Ԫ�ط��� | H | C | O | Cl | Ca | Fe | Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ӹ���ķ�����Һ�к���2~5%��NaNO2������һ�ֻ�����Ⱦ�������NH4Cl��Һ�������˷�����Һ��ʹNaNO2ת��Ϊ�����ʡ��÷�Ӧ���������У�
��һ����NaNO2+NH4Cl= NH4NO2+X
�ڶ�����NH4NO2 N2��+2H2O
N2��+2H2O
��1����һ����Ӧ��X�Ļ�ѧʽ�� ��
��2���ڶ�����Ӧ���ڻ�ѧ��Ӧ���������е� ��
��3��NH4NO2�е����⡢������Ԫ�ص��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij���ʼ��ȷֽ��������ͭ��ˮ��������ʺ��е�ȫ��Ԫ����
| A��Cu��O | B��Cu��O��H | C��O��H | D��Cu��H |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ԭ���о���Ԫ������������ǡ����������������������������������������� (�� )
| A�������� | B������ �� �� | C����������� | D�����ԭ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com