
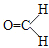
 ������̼����Ԫ�ص����������Ϊ
������̼����Ԫ�ص����������Ϊ| ���ԭ��������ԭ�Ӹ��� |
| ��Է������� |
| 2 |
| 30 |
| 2 |
| 18 |
| 1.5mg/L-0.5mg/L |
| 0.5mg/L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe2+��Ca2+��CO32-��Cl- |
| B��Na+��SO42-��Cl-��K+ |
| C��Ba2+��H+��Cl-��NO3- |
| D��Ag+��NH4+��NO3-��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ���������ǻ�ѧ����˼��ͷ�����ͷ���е��ۺ��������������ǻ�ѧѧϰ�����ս���ͶԻ�ѧ��ʶ����߾��磮 ��ˮ��֪ʶΪ������������Ļ�ѧ�ۣ���ش��������⣺
��ѧ���������ǻ�ѧ����˼��ͷ�����ͷ���е��ۺ��������������ǻ�ѧѧϰ�����ս���ͶԻ�ѧ��ʶ����߾��磮 ��ˮ��֪ʶΪ������������Ļ�ѧ�ۣ���ش��������⣺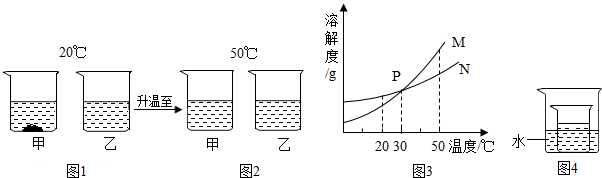
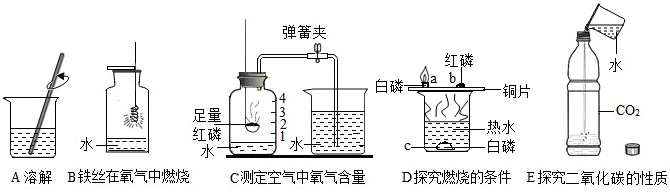
| 1 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
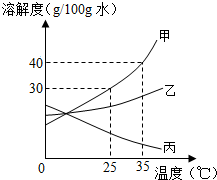 һͬѧ�ⶨ�˼ס��ҡ������ֹ������ʵ��ܽ�ȵı仯��ȡ�㻭�����ܽ��������ͼ��ʾ�����뵽��ʦ�����ܽ�����ߵ����֪ʶ���뵽�������⣬��ش�
һͬѧ�ⶨ�˼ס��ҡ������ֹ������ʵ��ܽ�ȵı仯��ȡ�㻭�����ܽ��������ͼ��ʾ�����뵽��ʦ�����ܽ�����ߵ����֪ʶ���뵽�������⣬��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
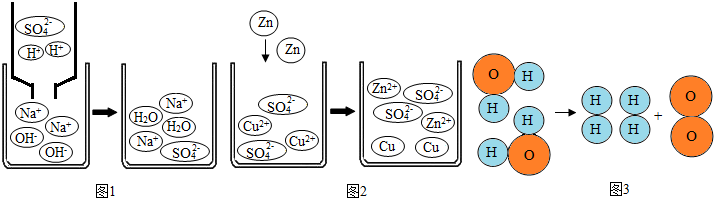
| A��ͼ1�ķ�Ӧ��������H+��OH-����ˮ�����Լ�Na+��SO42-����Na2SO4���ӵĹ��� |
| B��ͼ2�еķ�Ӧ��������пԭ�Ӻ�ͭ��������п���Ӻ�ͭԭ�ӵĹ��� |
| C��ͼ3�еķ�Ӧ�������Ƿ������ѳ�ԭ�ӣ�Ȼ��ԭ��������������·��ӵĹ��� |
| D���������漰�Ļ�ѧ��Ӧ���������и��ֽⷴӦ���û���Ӧ���ֽⷴӦ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com