ij����С���ͬѧ������װ��̽��������ʵ�����Ʒ���
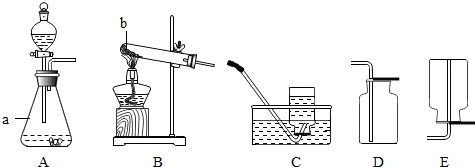
��1��д��ͼ�б�ŵ��������ƣ�a
��ƿ
��ƿ
��b
�Թ�
�Թ�
��
��2��д��ʵ������Bװ����ȡ�����Ļ�ѧ����ʽ
��
��3����С��ͬѧ��Bװ�ò���˳��
��װҩƷ�����û��˵������ʱ������ҩƷȡ��Ӧ
�����Թܵײ�
�����Թܵײ�
��
�������������̶��Թܣ�����װ�õ�˳����
�������ϣ�������
�������ϣ�������
���Թܿ���������б��ԭ����
��ֹ����ˮ���������Թ�����
��ֹ����ˮ���������Թ�����
��
�ۼ��ȣ�����ʱӦ��
Ԥ��
Ԥ��
��
���ռ����壮��Ҫ�õ��������������ѡ����ռ�װ��Ϊ
D
D
�����ţ����ø�װ���ռ����Բ�ȡ
�����ǵ�ľ������ƿ�ڸ�ȼ
�����ǵ�ľ������ƿ�ڸ�ȼ
��֤�������Ѿ��ռ����ˣ���Ҫ�õ���������������ѡ����ռ�װ����
C
C
�����ţ���
ʵ���������������ҳ�����ʵ�������©����һ��ʵ�鲽��
��װҩƷǰ���װ��������
��װҩƷǰ���װ��������
��
��4��ͨ���������Ͽ�֪�����ȹ���̼�����[NH
4HCO
3]����ȹ���̼������[NaHCO
3]���ܵõ�������̼���壬�仯ѧ����ʽ�ֱ��ǣ�
NH
4HCO
3NH
3��+H
2O+CO
2�� 2NaHCO
3Na
2CO
3+H
2O+CO
2��
ijͬѧ���ü���̼�����Ƶķ�����ȡCO
2��Ӧ��ѡ�õķ���װ����
B
B
�����ţ�������ѡ��̼�������ȡCO
2��������
ͬʱ���ɰ���������ռ����Ķ�����̼����
ͬʱ���ɰ���������ռ����Ķ�����̼����
��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�

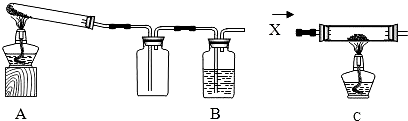
 ��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺
��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺