
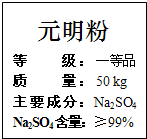
 Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ
Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ| 142 |
| x |
| 117 |
| y |
| 33 |
| 2.33g |
| 100g |
| 10g |
| 14.2g |
| 15g |
| 1.17g |
| 28.47g |


 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
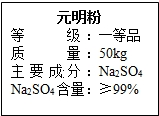 Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0g��Ʒ������ֻ�����������ʣ�����һ������ˮ�ܽ⣬���˵�100.0g��Һ��ȡ10.0g��Һ������10%��BaCl2��Һ20.8g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4+BaCl2=2NaCl+BaSO4����
Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0g��Ʒ������ֻ�����������ʣ�����һ������ˮ�ܽ⣬���˵�100.0g��Һ��ȡ10.0g��Һ������10%��BaCl2��Һ20.8g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4+BaCl2=2NaCl+BaSO4�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
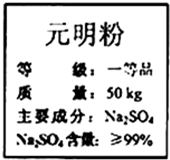 ���պ�����˳������â�����ǻ���Ψһ�ش�â������̽��������ߣ���Ԫ����Ʒλ�ߡ������ã����ܹ��������������Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0g��Ʒ������ֻ�����������ʣ�����һ������ˮ�ܽ⣬���˵�100.0g��Һ��ȡ10.0g��Һ������10%���Ȼ�����Һ20.8g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4+BaCl2=2NaCl+BaSO4����
���պ�����˳������â�����ǻ���Ψһ�ش�â������̽��������ߣ���Ԫ����Ʒλ�ߡ������ã����ܹ��������������Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0g��Ʒ������ֻ�����������ʣ�����һ������ˮ�ܽ⣬���˵�100.0g��Һ��ȡ10.0g��Һ������10%���Ȼ�����Һ20.8g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4+BaCl2=2NaCl+BaSO4�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
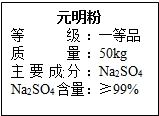
��12�֣�Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��
Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0 g��Ʒ������ֻ�����������ʣ�
����һ������ˮ�ܽ⣬���˵�100.0 g��Һ��ȡ10.0 g��Һ������10%��BaCl2��Һ20.8
g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4 + BaCl2 = 2NaCl + BaSO4����
��1������10.0 g��Һ��Na2SO4��������
��2��������һ�μ�⣬���㲢�ж���ƷNa2SO4�ĺ����Ƿ���ϱ�ǩҪ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0g��Ʒ������ֻ�����������ʣ�����һ������ˮ�ܽ⣬���˵�100.0g��Һ��ȡ10.0g��Һ������10%��BaCl2��Һ20.8g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4+BaCl2=2NaCl+BaSO4����
Ԫ��������Ҫ�Ļ���ԭ�ϣ���ͼ��ijƷ��Ԫ���۰�װ���ϵIJ��ֱ�ǩ��Ϊ�ⶨ��Ԫ������Na2SO4�����Ƿ���ϱ�ǩҪ��15.0g��Ʒ������ֻ�����������ʣ�����һ������ˮ�ܽ⣬���˵�100.0g��Һ��ȡ10.0g��Һ������10%��BaCl2��Һ20.8g��ǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽ��Na2SO4+BaCl2=2NaCl+BaSO4�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com