
��10����25��������������ֽ����ȼ��ϡ�ͼ���ҽҩ��Ʒ�ȵ���Ҫԭ�ϡ�ij�����ƴ�Ʒ�к����������Ȼ��ơ��Ȼ�þ��ʵ���ҽ����ᴿ���������£�
�ش��������⣺
��1�����������ƺ�̼���Ƴ���ʱ����Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ ��
��2���������ƺ�̼�������Ҫ���ӹ����ˣ��ɼ������� ��Һ��ȥ��
��3������������������Ҫ����ʱ������Ϊ�˻�ô����������ƣ�Ӧ������ ������ţ���
A.��ȫ����ʱֹͣ����
B.���ʱֹͣ���ȣ�������������
C.�д�����������ʱֹͣ���ȣ�������ȥʣ���������Һ
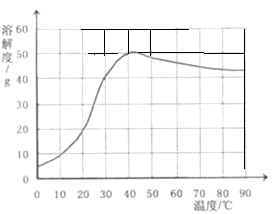
��4����֪�����Ƶ��ܽ�����¶ȱ仯����������ͼ��ʾ��40��ʱ��100g����ˮ���ܽ� g�����ƴﵽ���͡����ñ�����Һ�����¶���90�棬�۲쵽�������� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п����������ר���� ���ʵķ������ᴿ ���ͣ������
��10����25��������������ֽ����ȼ��ϡ�ͼ���ҽҩ��Ʒ�ȵ���Ҫԭ�ϡ�ij�����ƴ�Ʒ�к����������Ȼ��ơ��Ȼ�þ��ʵ���ҽ����ᴿ���������£�
�ش��������⣺
��1�����������ƺ�̼���Ƴ���ʱ����Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ ��
��2���������ƺ�̼�������Ҫ���ӹ����ˣ��ɼ������� ��Һ��ȥ��
��3������������������Ҫ����ʱ������Ϊ�˻�ô����������ƣ�Ӧ������ ������ţ���
A.��ȫ����ʱֹͣ����
B.���ʱֹͣ���ȣ�������������
C.�д�����������ʱֹͣ���ȣ�������ȥʣ���������Һ
��4����֪�����Ƶ��ܽ�����¶ȱ仯����������ͼ��ʾ��40��ʱ��100g����ˮ���ܽ� g�����ƴﵽ���͡����ñ�����Һ�����¶���90�棬�۲쵽�������� ��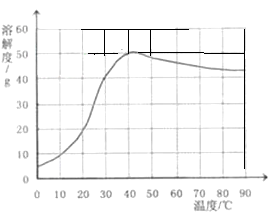
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п����������ר�������ʵķ������ᴿ ���ͣ������
��10����25��������������ֽ����ȼ��ϡ�ͼ���ҽҩ��Ʒ�ȵ���Ҫԭ�ϡ�ij�����ƴ�Ʒ�к����������Ȼ��ơ��Ȼ�þ��ʵ���ҽ����ᴿ���������£�
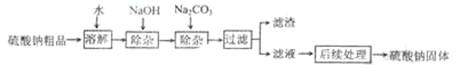
�ش��������⣺
��1�����������ƺ�̼���Ƴ���ʱ����Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ ��
��2���������ƺ�̼�������Ҫ���ӹ����ˣ��ɼ������� ��Һ��ȥ��
��3������������������Ҫ����ʱ������Ϊ�˻�ô����������ƣ�Ӧ������ ������ţ���
A.��ȫ����ʱֹͣ����
B.���ʱֹͣ���ȣ�������������
C.�д�����������ʱֹͣ���ȣ�������ȥʣ���������Һ
��4����֪�����Ƶ��ܽ�����¶ȱ仯����������ͼ��ʾ��40��ʱ��100g����ˮ���ܽ� g�����ƴﵽ���͡����ñ�����Һ�����¶���90�棬�۲쵽�������� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п�����ר����ר������ѧ����ʽ���㣨һ�� ���ͣ�������
��10�㶫ʡ25���Ȼ�����һ����Ҫ�Ļ���ԭ�ϣ�����Ȼ�����Һ���Ƶ���������
�����Ƶ����ʣ������Ļ�ѧ��Ӧ���£�2NaCl + 2H2O Cl2 ��+ H2
��+ 2NaOH����ȡ100g
Cl2 ��+ H2
��+ 2NaOH����ȡ100g
��������������Ϊ25%���Ȼ�����Һ���е�⣬һ��ʱ���ֹͣͨ�磬��������������7.1g��
����㣺
��1�����������������Ƕ��٣�ʣ���Ȼ��Ƶ������Ƕ��٣�
��2����Ӧ����Һ��NaOH�����������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com