
| ������������g�� | 7 |
| CuO������g�� | 20 |
| ����������g�� | 20 |
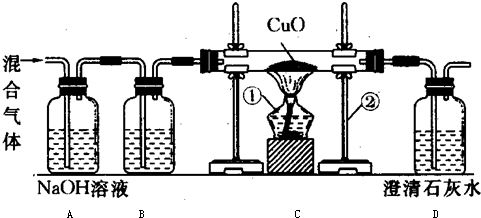
| 44 |
| 100 |
| x |
| 20g |
| ||
| 80 |
| 44 |
| y |
| 8.8g |
| 16g |
| 20g |


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?�ֿ���һģ��Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������
��2013?�ֿ���һģ��Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������| �Ͻ������/g | ϡ��������/mL | �������������/mL | |
| ��һ�� | 2 | 15 | 44.4 |
| �ڶ��� | 2 | 20 | 44.4 |
| ������ | 4 | 15 | 44.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
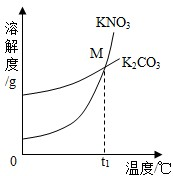 ��2013?��������ģ����1��K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȼ����ܽ��������ͼ��
��2013?��������ģ����1��K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȼ����ܽ��������ͼ��| �¶�/�� | 20 | 30 | 50 | 60 | 80 | |
| �ܽ��/g | K2CO3 | 110 | 114 | 121 | 126 | 139 |
| KNO3 | 31.6 | 45.8 | 85.5 | 110 | 169 | |
| ϡ���� |
| �� |
| Ũ���� |
| �� |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
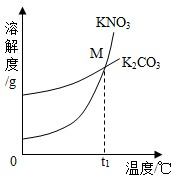 ��1��K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȼ����ܽ��������ͼ��
��1��K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȼ����ܽ��������ͼ��| �¶�/�� | 20 | 30 | 50 | 60 | 80 | |
| �ܽ��/g | K2CO3 | 110 | 114 | 121 | 126 | 139 |
| KNO3 | 31.6 | 45.8 | 85.5 | 110 | 169 | |
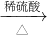 CuSO4
CuSO4 CuSO4[��֪Cu+2H2SO4��Ũ��
CuSO4[��֪Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O]
CuSO4+SO2��+2H2O]�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�ȼ����ܽ���������¡�
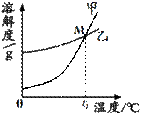 |
| �¶�/�� | 20 | 30 | 50 | 60 | 80 | |
| �ܽ��/g | K2CO3 | 110 | 114 | 121 | 126 | 139 |
| KNO3 | 31.6 | 45.8 | 85.5 | 110 | 169 |
��30��ʱ����124 g K2CO3�����м���100 gˮ������ܽ��������40�棬����Һ�����ʵ��������� ����������С�����䡱����
��������M������ ��
��2��ij����С�����÷�ͭ��ȡ����ͭ���������������������
| |||
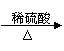 | |||
|
| |||
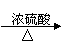 | |||
����������Cu CuSO4 [��֪Cu + 2H2SO4��Ũ���� CuSO4 + SO2��+2H2O]
����ɫ��ѧ�Ĺ۵����������Ϊ����_________������������ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�������������п���ѧ��ģ�Ծ��������棩 ���ͣ������
| �¶�/�� | 20 | 30 | 50 | 60 | 80 | |
| �ܽ��/g | K2CO3 | 110 | 114 | 121 | 126 | 139 |
| KNO3 | 31.6 | 45.8 | 85.5 | 110 | 169 | |
 CuSO4
CuSO4 CuSO4[��֪Cu+2H2SO4��Ũ��
CuSO4[��֪Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O]
CuSO4+SO2��+2H2O]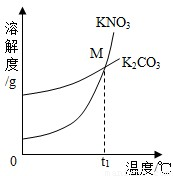
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com