
 ��ͼ��ʾ��ʵ��װ�ÿ������ⶨij����X����ɣ���ע����A��װ��0.32g����X������ͨ������������װ���������ȵ�����ͭ�IJ�����B��ʹ����X��ȫ��Ӧ���õ�����ʵ������ʵ��ǰB����20.32�ˣ�ʵ���B����20.00�ˣ�B���г��ֺ�ɫ��ĩ��C�����ռ�������ɫҺ����ˮ��ע����D���ռ������ɵĵ���0.28�ˣ�����˵����ȷ���ǣ�������
��ͼ��ʾ��ʵ��װ�ÿ������ⶨij����X����ɣ���ע����A��װ��0.32g����X������ͨ������������װ���������ȵ�����ͭ�IJ�����B��ʹ����X��ȫ��Ӧ���õ�����ʵ������ʵ��ǰB����20.32�ˣ�ʵ���B����20.00�ˣ�B���г��ֺ�ɫ��ĩ��C�����ռ�������ɫҺ����ˮ��ע����D���ռ������ɵĵ���0.28�ˣ�����˵����ȷ���ǣ�������| A�� | B���з����ķ�ӦΪ�û���Ӧ | |
| B�� | ����X�ǰ�����NH3�� | |
| C�� | �÷�Ӧ�����ɵ�ˮ�뵪���ķ��Ӹ�����Ϊ1��2 | |
| D�� | ����X�е�Ԫ������Ԫ�ص�������Ϊ7��1 |
���� ��������������ͭ��Ӧ����ͭ��������ˮ������X����ɣ��������������������е�Ԫ�ص�������B�������ļ���������ʧȥ����Ԫ�ص���������������ͭ����Ԫ���������Լ������������Ԫ�����������Լ����ˮ���������ټ���ˮ���Ӻ͵����ӵĸ����ȣ�
��� �⣺A��X�����к��е�Ԫ�ء���Ԫ�أ�����һ�����ǵ��ʣ�����ͭ���ڻ��������B���з�Ӧһ�������û���Ӧ����A����
B��ʵ��ǰB����20.32�ˣ�ʵ���B����20.00�ˣ���������ͭ����Ԫ������Ϊ0.32g����Ϊ��Ԫ�غ���Ԫ�ؽ������ˮ��������Ԫ������Ϊ��0.04g���ռ������ɵĵ���0.28�ˣ������е�Ԫ������Ϊ0.28g�����Ե�Ԫ������Ԫ��ԭ�Ӹ�����Ϊ��$\frac{0.28}{14}��\frac{0.04}{1}$=1��2���������岻�ǰ�������B����
C��ʵ��ǰB����20.32�ˣ�ʵ���B����20.00�ˣ���������ͭ����Ԫ������Ϊ0.32g����Ϊ��Ԫ�غ���Ԫ�ؽ������ˮ��������Ԫ������Ϊ��0.04g����������ˮ������Ϊ��0.32g+0.04g=0.36g���÷�Ӧ�����ɵ�ˮ�뵪���ķ��Ӹ�����Ϊ$\frac{0.36}{18}��\frac{0.28}{28}$=2��1����C����
D��ʵ��ǰB����20.32�ˣ�ʵ���B����20.00�ˣ���������ͭ����Ԫ������Ϊ0.32g����Ϊ��Ԫ�غ���Ԫ�ؽ������ˮ��������Ԫ������Ϊ��0.04g���ռ������ɵĵ���0.28�ˣ������е�Ԫ������Ϊ0.28g������X�е�Ԫ������Ԫ�ص�������Ϊ��0.28g��0.04g=7��1����D��ȷ��
��ѡD��
���� �����ؼ�����Ϥ����������ͭ��Ӧ���ɵ�ˮ�����͵��������Ĺ�ϵ��


 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | ������ | C�� | ̼���� | D�� | ������ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A����ѧ����Դ | B����ѧ��ũҵ |
| ��ú��ʯ���Dz���������Դ �ڷ��ܡ�̫�����������Դ | ����������ڸ��Ϸ� ������ʯ�Ҹ����������� |
| C����ѧ�뽡�� | D����ѧ�밲ȫ |
| ��������ȱ�ƻ�Ӱ������ �ڼ�����������Ӫ�����ǵ����� | ��ϡ��Ũ���ᣬ��ˮ����Ũ���� ��������Ȼ��й©Ӧ�����������Ȼ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Һ�������ڻ�����䣬��ΪҺ�����п�ȼ�� | |
| B�� | ϴ�Ӽ������ڳ����ۣ���Ϊϴ�Ӽ������黯���� | |
| C�� | �ɱ��������������Ϊ�ɱ�����ʱ���մ������� | |
| D�� | ��ʯ�ҿ����ڸ���������������Ϊ��ʯ����һ�ּ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯ�ҽ�ͿĨǽ�ں�����ˮ������ | |
| B�� | �ڿ��о�������ͷʱ�е�����ζ | |
| C�� | �øɱ�������������˹����� | |
| D�� | ʹ��ú����ȼ���շ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
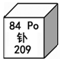
| A�� | Ԫ�ط���ΪPO | B�� | ������Ϊ84 | ||
| C�� | ���ԭ������Ϊ 209 | D�� | ���ڽ���Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com