
| ������; | ʯī���缫 | ���ʯ�и�� | ����̿��ˮ |
| ��Ӧ���� | ������ | Ӳ�ȴ� | ������ |
���� ���ʵ����ʾ������ʵ���;�����ʵ���;��ӳ���ʵ����ʣ����ݳ�����̼���ʵ����ʺ���;�����ش𣻸��ݼ���ȼ�յķ�Ӧд����Ӧ�Ļ�ѧ����ʽ�����ݶ�����̼�Ի�����Ӱ���Լ����ٶ�����̼�����Ĵ�ʩ��ɣ�
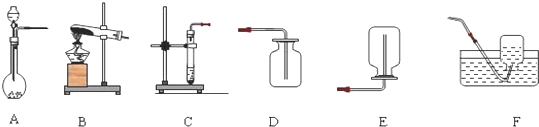
��� �⣺��1�����ڽ��ʯ��Ӳ�ȴ������и���ȣ����ڻ���̿�������ԣ������ھ�ˮ�����Ӳ�ȴ������ԣ�
��2��A��Һ̬������̼��������Ⱦ�������ϣ���ȷ��
B��������̼�ɸ�����ȼ������棬������������ȷ��
C��Һ̬������̼����ʱ���ȣ����ܽ��Ϳ�ȼ����Ż�㣬����
���AB��
��3����Ȼ���м�����ȼ�������˶�����̼��ˮ����ѧ����ʽΪCH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O��������̼�����ŷŻ���������ЧӦ�����Կ������õ�п��Դ��̫���ܡ�ˮ�ܵȣ���ֲ�����ֿ��Լ��ٶ�����̼���ŷţ��Ӷ�Ϊ��̼�������ף����CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O�����ң�ˮ�ܣ�ֲ�����֣�
���� ���ճ��������ʵ����ʺ���;�Լ���ѧ����ʽ����д��������ȷ�����Ĺؼ���


 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ϡ��������� | ��һ�μ���5g | �ڶ��μ���5g | �����μ���5g | ���Ĵμ���5g |
| ʣ���������� | 3g | 2g | l g | 1g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ľ̿��������ȼ�շ����� | |
| B�� | ��˿��������ȼ�վ��ң��������䣬���ɺ�ɫ���� | |
| C�� | ͭ˿�ڿ����м��ȱ�� | |
| D�� | ������ϡ��������Һ�������ݣ���Һ�����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com