
���� ���ʵ����ʾ������ʵ���;�����ݳ�����ѧ���ʵ����ʺ���;���з�����ɣ����ݻ�ѧʽ�������Լ���д������д�йصĻ�ѧ���T�ɣ�
��� �⣺��1���ٲ���ֲ������⣬������������ҽ����е��
�ڸɱ�������Ķ�����̼������ʱ���մ������ȣ��������˹����꣮
�۵�����ѧ���ʲ����ã���������������
�ܻ���̿���������ԣ�������������������ζ��
�ʴ�Ϊ���ٲ���֣��ڸɱ����۵������ܻ���̿��
��2���ٴ���ʯ����Ҫ�ɷ���̼��ƣ�
����Է���������С����������ˮ��
��2����������Ӿ�������������ӵ�ǰ���������2��
���Ȼ���������Ԫ�صĻ��ϼ���+2�ۣ�
�ʴ�Ϊ����CaCO3����H2O����2SO42-����$\stackrel{+2}{Fe}C{l}_{2}$��
���� �����ѶȲ������ճ��������ʵ������Լ���ѧʽ����д�ķ�������ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
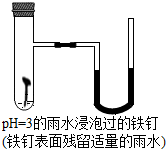 ��ͼ��̽������������ˮ������������ʴ��װ��ͼ��ʵ�鷢�ֿ�ʼʱU��Һ������Ҹߣ�һ��ʱ���Һ���Ϊ����ҵͣ����ݸ�ʵ����������˵�����Ͻ����ǣ�������
��ͼ��̽������������ˮ������������ʴ��װ��ͼ��ʵ�鷢�ֿ�ʼʱU��Һ������Ҹߣ�һ��ʱ���Һ���Ϊ����ҵͣ����ݸ�ʵ����������˵�����Ͻ����ǣ�������| A�� | ʵ�鿪ʼʱ������������� | |
| B�� | һ��ʱ����Թ��������屻��Ӧ | |
| C�� | ��ʵ����˵��������ı�Ҫ������ˮ������ | |
| D�� | U����Һ�����������ʾ������������ѹ�ı仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
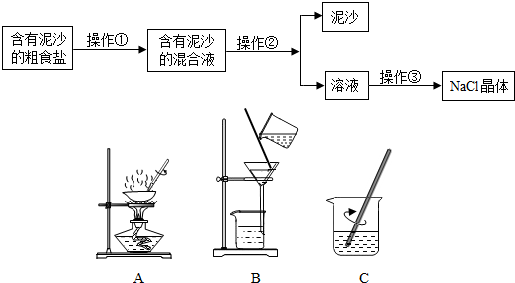
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 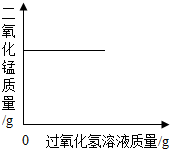 ��һ�����Ķ��������м������������Һ ��һ�����Ķ��������м������������Һ | |
| B�� | 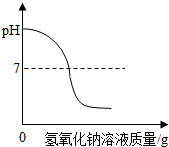 ��ϡ�����еμ�����������Һ ��ϡ�����еμ�����������Һ | |
| C�� | 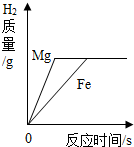 ��������ȫ��ͬ��ϡ�����зֱ����Mg��Fe ��������ȫ��ͬ��ϡ�����зֱ����Mg��Fe | |
| D�� | 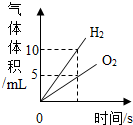 ��ˮͨ��һ��ʱ�� ��ˮͨ��һ��ʱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ͨ����ͬ״̬���ʼ䷴Ӧǰ�������ı仯�о������غ㶨�� | |
| B�� | ���ˮʵ���У�ͨ���������������о�ˮ����� | |
| C�� | ͨ���ı��ܼ����¶Ȳⶨ�����ܽ�Ķ��٣��о�Ӱ�������ܽ��Ե����� | |
| D�� | ���ù����������ܱ�������ȼ�յ�ʵ����֤������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com