
| ʵ�鲽�輰���� | ʵ������ | ʵ����� |
| ȡ��Ʒ���Թ��У�������������ˮ�����ã� ��ȡ�ϲ���Һ��������ɫ��̪��Һ �ڵ�ȥ�ϲ���Һ�������Թ���ע��ϡ���ᣮ | ������ɫ��̪��Һ��� �������ݲ��� | �ֱ��� |
| ������ɫ��̪��Һ����� �������ݲ��� | ����ȫ���� | |
| ������ɫ��̪��Һ��� ��û�����ݲ��� | ��û�б��� |
���� ��3�������������Ƶı��ʳ̶ȷ�������û�б��ʣ�ֻ�����������ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ����û�����ݲ�����
�ڲ��ֱ��ʣ������������ƺ�̼��ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��ȫ�����ʣ�ֻ����̼��ƣ��ӷ�̪��Һ���ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��4������̼��ƺ����ᷴӦ����������̼����д����صķ���ʽ��
��� �⣺��3�������������Ƶı��ʳ̶ȷ�������û�б��ʣ�ֻ�����������ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ����û�����ݲ�����
�ڲ��ֱ��ʣ������������ƺ�̼��ƣ��ӷ�̪��Һ�ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ�����
��ȫ�����ʣ�ֻ����̼��ƣ��ӷ�̪��Һ���ῴ����̪��Һ��ɺ�ɫ����ϡ���������ݲ������ʴ�Ϊ��
| ʵ�鲽�輰���� | ʵ������ | ʵ����� |
| �������ݲ��� | ���ֱ��� | |
| �������ݲ��� | ��ȫ���� | |
| ����ɫ��̪��Һ��� | û�б��� |
���� ���⿼�����������Ƶı��ʣ����������Ʊ��ʵ�ԭ����������ơ�̼��Ƶ������ǽ������ǰ��ͻ��������⣬��Ҫ��������������ۣ����߸��ݽ����Ƴ�������ȷ��д��ѧ����ʽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ; | ɱ������ |
| ��Ҫ�ɷ� | ���ᡢ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ǵ���ɵ����� | |
| B�� | ���ǹ������ʵĻ������� | |
| C�� | ���ǻ�ѧ�仯�е���С���� | |
| D�� | �������˶��Ķ�ԭ�ӡ������Ǿ�ֹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2�л���O2��ͨ�����ȵ�ľ̿�� | B�� | KOH�л���KCl��������ϡ���ᣩ | ||
| C�� | ϡ�����л������ᣨ�����ᱵ��Һ�� | D�� | CaO�л���CaCO3���������գ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
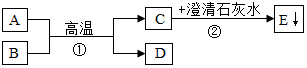 ��֪A��BΪ���ֺ�ɫ��ĩ��DΪ��ɫ���ʣ�A��B��C��D��E��������֮���ת����ϵ��ͼ��ʾ��ش�
��֪A��BΪ���ֺ�ɫ��ĩ��DΪ��ɫ���ʣ�A��B��C��D��E��������֮���ת����ϵ��ͼ��ʾ��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
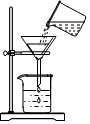 ������������ɳ��ʳ���ᴿ������ʳ��ˮ��Һ��ijͬѧ��������ʵ��������ش��������⣮
������������ɳ��ʳ���ᴿ������ʳ��ˮ��Һ��ijͬѧ��������ʵ��������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 A-��Ϊ���꼶��ѧ��ѧ�����ʣ���֪AΪ��������������������CΪ����ʯ����Ҫ�ɷ֣�DΪ��ɫ������EΪ��ɫ���嵥�ʣ�FΪdz��ɫ��Һ��F��G��I��Ϊֻ����һ�����ʵ���Һ����������ʾ���ʼ�������ת���Ĺ�ϵ������������ĩ���������ͼ��ʾ����ش���������
A-��Ϊ���꼶��ѧ��ѧ�����ʣ���֪AΪ��������������������CΪ����ʯ����Ҫ�ɷ֣�DΪ��ɫ������EΪ��ɫ���嵥�ʣ�FΪdz��ɫ��Һ��F��G��I��Ϊֻ����һ�����ʵ���Һ����������ʾ���ʼ�������ת���Ĺ�ϵ������������ĩ���������ͼ��ʾ����ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ȼ���̻� | B�� |  ������� | C�� |  ��ɽ�ڻ� | D�� |  ţ�̱��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com