
����Ŀ��ͬѧ�ǿ���ͨ�����з�ʽ��ʶ������
����ɽǶȡ�
�ٿ������������ԼΪ78%��������___________��
��Ϊ�ⶨ������������������������ͼʵ�顣
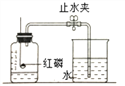
��.Ϊ��ȷ��ʵ��ɹ�����װҩƷ֮ǰӦ�ü��װ�õ� _________��
��.��ʵ���к�����Ҫ������ԭ����__________��
��.����ȼ�յ�������____________����Ӧ�Ļ�ѧ����ʽ ____________��
��.��ȴ�����º��ֹˮ�й۲쵽�������� _______________���ɴ˵ó��������������������ԼΪ ___________��
���۽Ƕ� ��
���á���ѧ���š���ͼʾ����ա�
![]()
ͼ ʾ |
| ______ |
|
��ѧ���� | ______ | N2 | _______ |
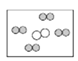
��ͬ��ͬѹ�£����������ȵ��ڷ��Ӹ����ȡ������Կ����������ɷ֣���ͼ�ɱ�ʾ������ģ�͵���__________(��ѡ��)��
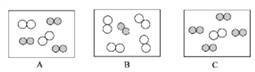
���仯�Ƕȡ�
��һ��������ѹ�£������в�����ֵķе����£�
��� | ���� | ���� | ������̼ |
�е�(��) | -195.8 | -183.0 | -78.4 |
�ٽ�ȼ�ŵ�ľ������ʢ�б�����ֵĻ��Һ�ĸ�ƿ�ڣ��۲쵽��������____________��
�����������������_____________��
A��ľ̿��������ȼ�գ�������
B����˿�ڿ�����ȼ�գ��������䣬���ɺ�ɫ����
C���ӱ������ó���ˮ��ƿ�������Һ�飬˵����������ˮ����
D�����ó���ʯ��ˮ���Լ�ƿ�ڱ���һ���Ĥ��֤���������ж�����̼
����˿��������ȼ�յĻ�ѧ����ʽ��____________��
��Ӧ�ýǶȡ�
�پƾ�(C2H5OH)��һ�ֳ������������ƾ���___________��Ԫ����ɣ�������Ԫ������Ԫ�ص�������Ϊ_______����Ԫ�ص���������Ϊ________(���÷�����ʾ)��ÿ���ƾ����Ӻ�________��ԭ�ӣ�46gC2H5OH�к�_________����ԭ�ӡ���ƽ�ƾ�ȼ�յĻ�ѧ����ʽ��ϵ������Ϊ_______��
��C2H5OH+��O2![]() ��CO2+��H2O
��CO2+��H2O
��ʳƷ��װ�ڳ�N2�Է�������ΪN2�Ļ�ѧ����_____________��
���𰸡� N2 ���� ������ �ѹ��ƿ�е������������ ����ȼ�գ�����Ũ����̣����� 4P+5O2![]() 2P2O5 �ձ��ڵ�ˮ������ƿ�����̶�1��(�����÷�) 1/5 2N
2P2O5 �ձ��ڵ�ˮ������ƿ�����̶�1��(�����÷�) 1/5 2N ![]() 2NO2 C ȼ�ŵ�ľ��Ϩ�� B 3Fe��2O2
2NO2 C ȼ�ŵ�ľ��Ϩ�� B 3Fe��2O2![]() Fe3O4 3 3:8 3/23��13.0% 9 6.02��1023 1��3��2��3 �Ƚ��ȶ�
Fe3O4 3 3:8 3/23��13.0% 9 6.02��1023 1��3��2��3 �Ƚ��ȶ�
������������ɽǶȡ��ٿ������������ԼΪ78%�������ǵ������ڢ�.Ϊ��ȷ��ʵ��ɹ�����װҩƷ֮ǰӦ�ü��װ�õ������ԣ���.��ʵ���к�����Ҫ������ԭ���ǰ������ľ�����.����ȼ�ղ����������������������ף���Ӧ�Ļ�ѧ����ʽΪ��4P+5O2![]() 2P2O5����.��ȴ�����º��ֹˮ�й۲쵽���������ձ��ڵ�ˮ������ƿ�����̶�1�����ɴ˵ó��������������������ԼΪ1/5�����۽Ƕ� ��
2P2O5����.��ȴ�����º��ֹˮ�й۲쵽���������ձ��ڵ�ˮ������ƿ�����̶�1�����ɴ˵ó��������������������ԼΪ1/5�����۽Ƕ� ��
��������ѧ����������ͼʾ����ա�
![]()
ͼ ʾ |
| __ |
|
��ѧ���� | __2N____ | N2 | ____2NO2 ___ |
��ͬ��ͬѹ�£����������ȵ��ڷ��Ӹ����ȡ������Կ����������ɷ֣�����Լռ1/5������Լռ4/5���ʿɱ�ʾ������ģ�͵���
 �����仯�Ƕȡ��ٸû��Һ�е����е���ͣ����ȷ���������ʽ�ȼ�ŵ�ľ������ʢ�б�����ֵĻ��Һ�ĸ�ƿ�ڣ��۲쵽��������ȼ�ŵ�ľ��Ϩ������A��ľ̿��������ȼ�գ�����������ȷ��B����˿�ڿ����в���ȼ�գ�����C���ӱ������ó���ˮ��ƿ�������Һ�飬˵����������ˮ��������ȷ��D�����ó���ʯ��ˮ���Լ�ƿ�ڱ���һ���Ĥ��֤���������ж�����̼����ȷ������˿��������ȼ�ջ������䣬����������������ѧ����ʽ����3Fe��2O2
�����仯�Ƕȡ��ٸû��Һ�е����е���ͣ����ȷ���������ʽ�ȼ�ŵ�ľ������ʢ�б�����ֵĻ��Һ�ĸ�ƿ�ڣ��۲쵽��������ȼ�ŵ�ľ��Ϩ������A��ľ̿��������ȼ�գ�����������ȷ��B����˿�ڿ����в���ȼ�գ�����C���ӱ������ó���ˮ��ƿ�������Һ�飬˵����������ˮ��������ȷ��D�����ó���ʯ��ˮ���Լ�ƿ�ڱ���һ���Ĥ��֤���������ж�����̼����ȷ������˿��������ȼ�ջ������䣬����������������ѧ����ʽ����3Fe��2O2![]() Fe3O4����Ӧ�ýǶȡ��پƾ�(C2H5OH)��һ�ֳ������������ƾ���C��H��O����Ԫ����ɣ�������Ԫ������Ԫ�ص�������Ϊ
Fe3O4����Ӧ�ýǶȡ��پƾ�(C2H5OH)��һ�ֳ������������ƾ���C��H��O����Ԫ����ɣ�������Ԫ������Ԫ�ص�������Ϊ![]() ��ÿ���ƾ����Ӻ�9��ԭ�ӣ�46gC2H5OH�к���ԭ�ӵĸ���Ϊ
��ÿ���ƾ����Ӻ�9��ԭ�ӣ�46gC2H5OH�к���ԭ�ӵĸ���Ϊ![]() 6.02��1023=6.02��1023���ƾ�ȼ�յĻ�ѧ����ʽΪ��
6.02��1023=6.02��1023���ƾ�ȼ�յĻ�ѧ����ʽΪ��
C2H5OH+3O2![]() 2CO2+3H2O����ʳƷ��װ�ڳ�N2�Է�������ΪN2�Ļ�ѧ���ʱȽ��ȶ���
2CO2+3H2O����ʳƷ��װ�ڳ�N2�Է�������ΪN2�Ļ�ѧ���ʱȽ��ȶ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ǽ��п�ѧ̽������Ҫ;����
��1��ʵ��������ȡ����ʱ�ز����ٵIJ����� _____________��
��2������(NH3)��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С����������ˮ����ˮ�Լ��ԡ�ʵ����������Ȼ��(NH4Cl)����ʯ�����ֹ���Ļ�������ȡ������ͬʱ�����Ȼ��ƺ�ˮ��
��ʵ������ȡ�����Ļ�ѧ����ʽΪ________________��

����Ҫ��ȡNH3��Ӧ��ѡ����ͼ�ķ���װ����________������ĸ������Ҫ�ռ��������NH3��Ӧ��ѡ�������ĵ��ܽӿڴ����ҵ�����˳��Ϊ������װ�ó��ڽ�________��________��________��
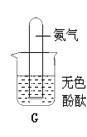
����Gͼ�������ռ��������Ĵ��Թܵ����ڵ�����ɫ��̪��ˮ�У��۲쵽��������_________________��
��3��ij�������ų��ķ�Һ�к���AgNO3��Zn(NO3)2��Cu(NO3)2�������ʣ�ij��ѧ����С���ͬѧ���������ʵ�鷽����

��д������һ�Ļ�ѧ����ʽ��_______________��
��������Y��ϡ���������ݲ�������ҺB�е����������______�֡�
����ҺA������_________�����������������������Һ��������
��4��С��ʵ��������Ե���Ŀ�ǣ�����һ����������������̼������Һ���ⶨ�����ȡ�����ʱ��С������������ͼ��ʾ�IJ���:����֪̼������Һ�ʼ��ԣ�

������д������������ƣ�a.___________��b.__________��
������ʵ�����ȷ����˳��Ϊ ________________���������ĸ��ʾ����
����ͼ��ʾ�����У���C������Ϊ12g������Ķ���Ϊ2.5g����̼����ʵ������Ϊ______g��
��С���ڲⶨ��������Һ������ʱ���Ƚ�pH��ֽ������ˮʪ���ٽ��вⶨ��������Һ��pHֵ_________��ѡ�ƫ����ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ�������з�̪��Һ����ֽ�����Թ��У��Թܿ���һ����֬�ޡ�(��ʾ����̪����ˮ���ɫ)������A��ȡŨ��ˮ��������֬����(10��15��)
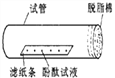
(1)A��������________������;��__________��
(2)ʵ���У��۲쵽��������_________________����˵���� _______________��
(3)ʵ���У��������Թ��·�һ�Ű�ֽ����ֽ������_______________��
(4)ijͬѧ����ʵ��ʱ��������ֽ����û�б�ɫ�����Թ�����������֬��ȴ����˺�ɫ����������������Ĵ������������___________________________����һʵ�黹˵���������Լ���_____________���лӷ��ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʯ��ʯ�dz��õĽ������ϡ�
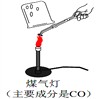
�ټ���ͬѧ���������ʵ�鷽������̽����
��.��ͬѧȡʯ��ʯ����ͼ��ʾ����ʵ�飨ú�����ܴﵽʯ��ʯ�ֽ���¶ȣ����۲쵽�ձ��ڱ�ʯ��ˮ����ǣ��ɴ˼�ͬѧ��Ϊʯ��ʯ�ѷֽ⡣��ͬѧ���۲�������������__________��
��.��ͬѧ��������ʵ�飬����±�
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ�������պ�������Թ��У���ˮ�����ˣ�ȡ��Һ���μ�____________��Һ | ��Һ��� | ֤�������� ����________ |
ȡ�����μ�������ϡ���� | _______ | ����̼��� |
��Ϊ�˲ⶨʯ��ʯ��̼��Ƶ������������������ʲ��μӷ�Ӧ������ͬѧ��Ƴ�ȡһ��������ʯ��ʯ��Ʒ�������������������ٸı䡣ʵ���й���������仯���£�
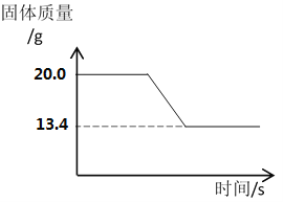
��.���ɶ�����̼��������____________g��[
��.��ʯ��ʯ��̼��Ƶ����ʵ����������ݻ�ѧ����ʽ��д��������̣�___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������һ����������������NaCl��Һ������������ʵ��������
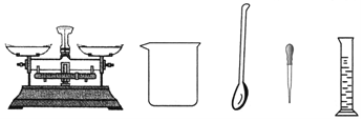
����գ�
������ʱ�ɰ�����ʵ�鲽����У������������_______���ܽ⡣
�Ƴ���ʱ��NaCl�����ĩӦ����������ƽ_________(����̡������̡�)��ֽƬ�ϡ�
�����ƹ�����Ϊ�ٽ�NaCl���ܽ⣬����Ҫ��һ�ֲ���������_______(����������)��
����ȡһ�������ˮ����������Ͳ�⣬����Ҫ��____________(����������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��С�����ּ���һö��ָ������ͭ�̣�����ͬѧ������ö��ָչ�����о���ѧϰ��
![]() ��������
��������![]() ����ڿ����в������⣬����ͭ�̵ġ����ָ������Ϊͭп�Ͻ�ͭ����¶���ڳ�ʪ�Ŀ�����������ͭ�̣�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ����ʽ̼��ͭ�����ֽ�����CuO��
����ڿ����в������⣬����ͭ�̵ġ����ָ������Ϊͭп�Ͻ�ͭ����¶���ڳ�ʪ�Ŀ�����������ͭ�̣�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ����ʽ̼��ͭ�����ֽ�����CuO��![]() ��
��![]() ��
��
���������Ͽ���֪����ʽ̼��ͭ��______��Ԫ����ɣ�
![]() ʵ��̽��
ʵ��̽��![]() ����ö��ָ�������ϡ�����У������ݲ�������Һ����ɫ��Ϊ����ɫ��
����ö��ָ�������ϡ�����У������ݲ�������Һ����ɫ��Ϊ����ɫ��
![]() С����Ϊ�������г��˺���
С����Ϊ�������г��˺���![]() �������ܺ�������______
�������ܺ�������______![]() ʵ���ҳ���______�ķ����������ֿ��ܺ��е����壮
ʵ���ҳ���______�ķ����������ֿ��ܺ��е����壮
![]() С����Ϊ����ɫ��Һ�е�����ֻ���Ȼ�п
С����Ϊ����ɫ��Һ�е�����ֻ���Ȼ�п![]() С����Ϊ��Һ�е����ʳ����Ȼ�п�⣬
С����Ϊ��Һ�е����ʳ����Ȼ�п�⣬
��Ӧ�ú���______��______![]() ��ȡ������������ɫ��Һ�������������Ƭ���۲쵽������
��ȡ������������ɫ��Һ�������������Ƭ���۲쵽������ ![]() ______��
______�� ![]() ______��֤ʵ���Լ��Ĺ۵㣮
______��֤ʵ���Լ��Ĺ۵㣮
![]() С��ȡ�������Ƶ�
С��ȡ�������Ƶ�![]() ��Һ������п����һ��ʱ�����Һ��ɫ��dz
��Һ������п����һ��ʱ�����Һ��ɫ��dz![]() ���С����ʵ�����֪������п��ͭ���ֽ����Ļ��������ǿ��˳����______��
���С����ʵ�����֪������п��ͭ���ֽ����Ļ��������ǿ��˳����______��
![]() С�����һ��̽�������ָ����ͭԪ�صĺ�����ȡһöͬ���ʵġ����ָ�����Ƶ�����Ϊ
С�����һ��̽�������ָ����ͭԪ�صĺ�����ȡһöͬ���ʵġ����ָ�����Ƶ�����Ϊ![]() ����ʦ��ָ���£��������ָ����Ũ������������Ȳ��账�������յõ�����������ͭ���Ƶ�������ȻΪ
����ʦ��ָ���£��������ָ����Ũ������������Ȳ��账�������յõ�����������ͭ���Ƶ�������ȻΪ![]() ʵ�������ͭԪ����ʧ���Բ���
ʵ�������ͭԪ����ʧ���Բ���![]() ���ָ����ͭԪ�ص����������Ƕ��٣�____________д���������
���ָ����ͭԪ�ص����������Ƕ��٣�____________д���������![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ܴﵽʵ��Ŀ����![]()
A. ����˿������ף����Եزⶨ�����������ĺ���
B. ��25mL����ˮ��25mL�ƾ�����50mL�ƾ���Һ
C. ������ͨ����������������Һ����ȥ![]() ��������CO
��������CO
D. ��ϡ������ڽ������棬������ƽ�ͼٻƽ�![]() ͭп�Ͻ�
ͭп�Ͻ�![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���й������IJ���֪ʶ����ͼ(��Ӧ��������ʡ��)�����ó���֪ʶ�ش�
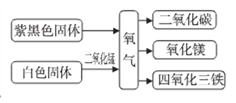
(1)�Ϻ�ɫ������_______(�ѧʽ)
(2)����þ�еĽ���Ԫ����_______(��Ԫ�ط���)
(3)��̬������̼�׳�_______
(4)MnO2��MnԪ�صĻ��ϼ�Ϊ_______�������ӵķ���Ϊ_______��
(5)ʵ�����ð�ɫ�����������Ļ�ѧ����ʽ__________________�����ж��������ڸ÷�Ӧ�е�������_________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ȼ��ص��ܽ��������ͼ��ʾ����ش��������⡣
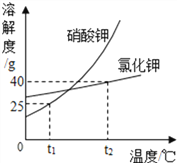
(1)t1��ʱ�����������ܽ�Ƚϴ����________;
(2)t2��ʱ�������ʵĵ�����������Һ������t1�棬���ˣ������������Һ����__________�����Ȼ�����Һ����;(������������������������)
(3)t2 ��ʱ����100gˮ����ʢ��50g�Ȼ��ص��ձ��У�����ܽ�õ���Һ����Ϊ_______g;
(4)���������ʵ���������Ϊ20%���������Һ��Ӧ������¶ȷ�Χ��__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com