
Ϊ�ⶨij̼����炙����еĺ�������С����ʵ������ȡ��8.5 g�û�����Ʒ��20 g NaOH��Һ���ȣ�ǡ����ȫ��Ӧ����ò�����Ϊ26.8 g(��ʾ����Ӧ�Ļ�ѧ����ʽΪNH4HCO3��2NaOH=Na2CO3��2H2O��NH3�������ɵİ���ȫ���ݳ��������ɷֲ�������Ҳ���μӷ�Ӧ)������
(1)���ɰ�����������
(2)�û����е�Ԫ�ص���������(��������ȷ��0.1%)��
(3)������Һ����������������
(1)1.7 g (2)16.5% (3)40%
��������(1)���������غ㶨�ɣ���������ɰ���������Ϊ8.5 g��20 g��26.8 g��1.7 g��
(2)��μӷ�Ӧ��̼����淋�����Ϊx���μӷ�Ӧ���������Ƶ�����Ϊy
NH4HCO3��2NaOH=Na2CO3��2H2O��NH3��
79?????? 80????????????????? 17
x??????? y?????????????????? 1.7 g
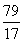 ��
��
x��7.9 g
̼����狀���Ԫ�ص�����Ϊ7.9 g��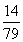 ��1.4 g
��1.4 g
̼����炙����е�Ԫ�ص���������Ϊ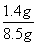 ��100%��16.5%
��100%��16.5%
(3) 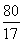 ��
��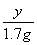
y��8 g
NaOH��Һ��������������Ϊ��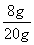 ��100%��40%
��100%��40%



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨij̼����炙����еĺ�������С����ʵ������ȡ��8.5 g�û�����Ʒ��20 g NaOH��Һ���ȣ�ǡ����ȫ��Ӧ����ò�����Ϊ26.8 g(��ʾ����Ӧ�Ļ�ѧ����ʽΪNH4HCO3+2NaOH====Na2CO3+2H2O+NH3�������ɵİ���ȫ���ݳ��������ɷֲ�������Ҳ���μӷ�Ӧ)������
(1)���ɰ�����������
(2)�û����е�Ԫ�ص���������(��������ȷ��0.1%)��
(3)������Һ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com