
Ä³ŃŠ¾æŠ”×éÓū¼ģŃé²ŻĖį¾§Ģåѳʷ·Ö½ā²śĪļ²¢²ā¶ØĘ÷ÖŹĮæ·ÖŹż£Ø¼ŁÉčŌÓÖŹ²»²ĪÓė·“Ó¦£©£®²ŻĖį¾§Ģå£ØH2C2O4•2H2O£©µÄĄķ»ÆŠŌÖŹ¼ū±ķ£®
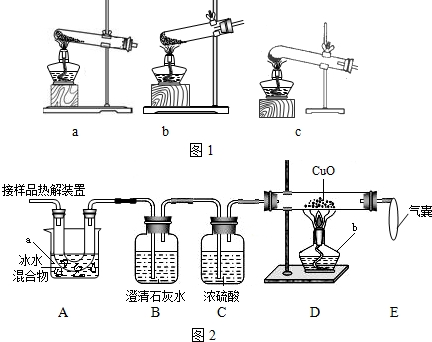
£Ø1£©¼ÓČČ·Ö½ā²ŻĖį¾§Ģå×īŹŹŅĖµÄ×°ÖĆŹĒ £ØĢīĶ¼1×ÖÄøŠņŗÅ£©£®

£Ø2£©Ķ¼2ŹĒŃéÖ¤ČČ·Ö½ā²śĪļÖŠŗ¬CO£¬CO2µÄ×°ÖĆ
¢ŁŅĒĘ÷aŗĶbµÄĆū³Ę·Ö±šŹĒ ŗĶ £®
¢ŚÖ¤Ć÷“ęŌŚCO2µÄĻÖĻóŹĒ £¬Ö¤Ć÷“ęŌŚCOµÄĻÖĻóŹĒ £¬DÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £®
¢Ū×°ÖĆAµÄ×÷ÓĆŹĒ £¬ĘųÄŅµÄ×÷ÓĆŹĒ £®
£Ø3£©ĪŖ²ā¶Øѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮ½ÖÖČēĻĀ·½°ø£®
|
ČŪµć |
·Šµć |
ČČĪČ¶ØŠŌ |
Óė¼ī·“Ó¦ |
|
101”ćC”«102”ćC |
150”ćC”«160”ćCÉż»Ŗ |
100.1”ćCŹ§Č„½į¾§Ė®£¬175”ćC·Ö½ā³ÉCO2£¬CO£¬H2O |
ÓėCa£ØOH£©2·“Ó¦²śÉś°×É«³Įµķ£ØCaC2O4£© |
¢Ł³ĘŅ»¶ØĮæѳʷÓĆÉĻĶ¼×°ÖĆ½ųŠŠŹµŃ飬²āµĆ×°ÖĆD·“Ó¦Ē°ŗóµÄÖŹĮæ²ī£¬ÓÉ“Ė¼ĘĖć³öµÄŹµŃé½į¹ū±ČŹµ¼ŹÖµĘ«µĶ£¬ÅųżŅĒĘ÷ŗĶ²Ł×÷µÄŅņĖŲ£¬ĘäŌŅņæÉÄÜÓŠ£ŗCOĪ“ĶźČ«·“Ó¦”¢ £®
¢Ś³ĘČ”8.75g²ŻĖį¾§ĢåѳʷÅäÖĘ50.00gČÜŅŗ£¬Č”10.00gČÜŅŗ¼ÓŹŹĮæµÄĻ”ĮņĖį£¬Č»ŗóµĪ¼Ó25.00g3.16%KMnO4ČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«£®
£ØŅŃÖŖ£ŗ2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2”ü+8H2O£©ŌņKMnO4ČÜŅŗĻŌ É«£¬25.00g3.16%KMnO4ČÜŅŗÖŠKMnO4µÄÖŹĮæ g£®Ēė¼ĘĖćѳʷ֊µÄÖŹĮæ·ÖŹż£®[Š“³ö¼ĘĖć¹ż³Ģ£¬M2£ØH2C2O4£©=90£¬M2£ØH2C2O4•2H2O£©=126£¬M2£ØKMnO4£©=158]£®
£Ø1£©c
£Ø2£©¢ŁÉÕ±”¢¾Ę¾«µĘ
¢ŚBÖŠ³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£»DÖŠŗŚÉ«¹ĢĢå±ä³ÉŗģÉ«
CO+CuO Cu+CO2
Cu+CO2
¢Ū³żČ„²ŻĖįÕōĘū£¬·ĄÖ¹¶Ō¶žŃõ»ÆĢ¼µÄ¼ģŃé²śÉśøÉČÅ£»ŹÕ¼ÆŅ»Ńõ»ÆĢ¼£¬·ĄÖ¹ĪŪČ¾æÕĘų
£Ø3£©¢ŁÉś³ÉµÄĶÓÖ±»Ńõ»Æ ¢Ś×Ļŗģ 0.79g 90%
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©²ŻĖįµÄČŪµć½ĻµĶ£¬ŹÜČČČŻŅ×ČŪ»Æ£¬ÓĆc×°ÖĆ¼ÓČČ²ŻĖįŹ±²»ČŻŅ×ĻĀĮ÷£¬ŹŹŅĖÓĆĄ“¼ÓČČ²ŻĖį£®
¹ŹĢī£ŗc£®
£Ø2£©¢ŁŅĒĘ÷aŗĶbµÄĆū³Ę·Ö±šŹĒÉÕ±”¢¾Ę¾«µĘ£®
¹ŹĢī£ŗÉÕ±£»¾Ę¾«µĘ£®
¢ŚÖ¤Ć÷“ęŌŚCO2µÄĻÖĻóŹĒ£ŗBÖŠ³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£»Ö¤Ć÷“ęŌŚCOµÄĻÖĻóŹĒ£ŗDÖŠŗŚÉ«¹ĢĢå±ä³ÉŗģÉ«£®
¹ŹĢī£ŗBÖŠ³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£»DÖŠŗŚÉ«¹ĢĢå±ä³ÉŗģÉ«£®
DÖŠŃõ»ÆĶŗĶŅ»Ńõ»ÆĢ¼ŌŚ¼ÓČČŹ±·“Ó¦ÄÜÉś³ÉĶŗĶ¶žŃõ»ÆĢ¼£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗCO+CuO Cu+CO2£®
Cu+CO2£®
¹ŹĢī£ŗCO+CuO Cu+CO2£®
Cu+CO2£®
¢Ū×°ÖĆAµÄ×÷ÓĆŹĒ£ŗ³żČ„²ŻĖįÕōĘū£¬·ĄÖ¹¶Ō¶žŃõ»ÆĢ¼µÄ¼ģŃé²śÉśøÉČÅ£»ĘųÄŅµÄ×÷ÓĆŹĒ£ŗŹÕ¼ÆŅ»Ńõ»ÆĢ¼£¬·ĄÖ¹ĪŪČ¾æÕĘų£®
¹ŹĢī£ŗ³żČ„²ŻĖįÕōĘū£¬·ĄÖ¹¶Ō¶žŃõ»ÆĢ¼µÄ¼ģŃé²śÉśøÉČÅ£»ŹÕ¼ÆŅ»Ńõ»ÆĢ¼£¬·ĄÖ¹ĪŪČ¾æÕĘų£®
£Ø3£©¢ŁŅ»Ńõ»ÆĢ¼²æ·Ö·“Ó¦£¬Éś³ÉµÄĶÖŲŠĀ±»Ńõ»ÆµČŅņĖŲ¶¼Äܹ»µ¼ÖĀ¼ĘĖć³öµÄŹµŃé½į¹ū±ČŹµ¼ŹÖµĘ«µĶ£®
¹ŹĢī£ŗÉś³ÉµÄĶÓÖ±»Ńõ»Æ£®
¢ŚøßĆĢĖį¼ŲČÜŅŗŹĒŃÕÉ«×ĻŗģÉ«µÄ£®
¹ŹĢī£ŗ×Ļŗģ£®
25.00g3.16%KMnO4ČÜŅŗÖŠKMnO4µÄÖŹĮæĪŖ£ŗ25.00g”Į3.16%=0.79g£®
¹ŹĢī£ŗ0.79£®
Éč10.00gČÜŅŗÖŠŗ¬²ŻĖį¾§ĢåµÄÖŹĮæĪŖX£¬
ÓÉ2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2”ü+8H2OæÉÖŖ£¬
5H2C2O4•2H2O”ś5H2C2O4”ś2KMnO4£¬
630 316
X 0.79g
 =
=
X=1.575g£¬
50.00gČÜŅŗÖŠŗ¬²ŻĖį¾§ĢåµÄÖŹĮæĪŖ£ŗ1.575g”Į5=7.875g£¬
²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹżĪŖ£ŗ ”Į100%=90%£¬
”Į100%=90%£¬
“š£ŗѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹżĪŖ90%£®
æ¼µć£ŗŹµŃéĢ½¾æĪļÖŹµÄ×é³É³É·ÖŅŌ¼°ŗ¬Į棻³£¼ūĘųĢåµÄ¼ģŃéÓė³żŌÓ·½·Ø£®
µćĘĄ£ŗ±¾ĢāÉę¼°»Æѧ·½³ĢŹ½µÄŹéŠ“”¢ŹµŃéĻÖĻóµÄÅŠ¶Ļ”¢øł¾Ż»Æѧ·½³ĢŹ½½ųŠŠ¼ĘĖćµČ·½ĆęµÄÖŖŹ¶£¬ŹĒµäŠĶµÄ×ŪŗĻĢā£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| ČŪµć | ·Šµć | ČČĪČ¶ØŠŌ | Óė¼ī·“Ó¦ |
| 101”ćC”«102”ćC | 150”ćC”«160”ćCÉż»Ŗ | 100.1”ćCŹ§Č„½į¾§Ė®£¬175”ćC·Ö½ā³ÉCO2£¬CO£¬H2O | ÓėCa£ØOH£©2·“Ó¦²śÉś°×É«³Įµķ£ØCaC2O4£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğø£½ØŹ”ĻĆĆÅŹŠ¾ÅÄź¼¶ÉĻѧʌ֏Įæ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢ½¾æĢā
Ä³ŃŠ¾æŠ”×éÓū¼ģŃé²ŻĖį¾§Ģåѳʷ·Ö½ā²śĪļ£¬²¢²ā¶Øѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹż£Ø¼ŁÉčŌÓÖŹ²»²ĪÓė·“Ó¦£©”£ŅŃÖŖ£ŗÅØĮņĖįæÉ×÷ĪŖøÉŌļ¼Į£»²ŻĖį¾§Ģå£ØH2C2O4”¤2H2O £©µÄŠŌÖŹ¼ūĻĀ±ķ£ŗ
|
ČŪµć |
·Šµć |
ČČĪČ¶ØŠŌ |
ĘäĖū |
|
101”ꔫ102”ę |
150”ꔫ160”ęÉż»Ŗ |
100.1”ę·Ö½ā³öĖ®£¬175”ę·Ö½ā³ÉCO2”¢CO”¢H2O |
Óė Ca(OH)2·“Ó¦²śÉś°×É«³Įµķ(CaC2O4) |
£Ø1£©Ķ¼ 1 ŹĒ¼ÓČČ×°ÖĆ”£×īŹŹŅĖµÄ¼ÓČČ·Ö½ā²ŻĖį¾§Ģå×°ÖĆŹĒC”£ČōŃ”×°ÖĆ a æÉÄÜ»įŌģ³ÉµÄŗó¹ūŹĒ_____________________£»ČōŃ”×°ÖĆBæÉÄÜ»įŌģ³ÉµÄŗó¹ūŹĒ_________________”£

£Ø2£©Ķ¼ 2 ŹĒŃéÖ¤ČČ·Ö½ā²śĪļÖŠŗ¬ CO ”¢ CO2µÄ×°ÖĆ”£

¢Ł ×°ÖĆ A µÄ×÷ÓĆŹĒ_____________________£¬ĘųÄŅµÄ×÷ÓĆŹĒ_____________________”£
¢Ś Ö¤Ć÷“ęŌŚ CO2µÄĻÖĻóŹĒ______________£¬Ö¤Ć÷“ęŌŚ CO µÄĻÖĻóŹĒ______________”£
£Ø3£©ĪŖ²ā¶Øѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹż£¬Éč¼ĘČēĻĀ·½°ø£ŗ³ĘČ”Ņ»¶ØĮæѳʷ£¬ÓĆÉĻŹö×°ÖĆ½ųŠŠŹµŃ飬³ĘĮæ×°ÖĆD·“Ó¦Ē°ŗóµÄÖŹĮæ²ī”£ÓÉ“Ė¼ĘĖć³öµÄŹµŃé½į¹ū±ČŹµ¼ŹÖµĘ«µĶ£¬ÅųżŅĒĘ÷ŗĶ²Ł×÷ŅņĖŲ£¬ĘäæÉÄÜŌŅņ£ŗ_________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³ŃŠ¾æŠ”×éÓū¼ģŃé²ŻĖį¾§Ģåѳʷ·Ö½ā²śĪļ²¢²ā¶ØĘ÷ÖŹĮæ·ÖŹż£Ø¼ŁÉčŌÓÖŹ²»²ĪÓė·“Ó¦£©£®²ŻĖį¾§Ģå£Ø H2C2O4•2H2O£©µÄĄķ»ÆŠŌÖŹ¼ū±ķ£®
£Ø1£©¼ÓČČ·Ö½ā²ŻĖį¾§Ģå×īŹŹŅĖµÄ×°ÖĆŹĒ”” ””£ØĢīĶ¼1×ÖÄøŠņŗÅ£©£®

£Ø2£©Ķ¼2ŹĒŃéÖ¤ČČ·Ö½ā²śĪļÖŠŗ¬CO£¬CO2µÄ×°ÖĆ
¢ŁŅĒĘ÷aŗĶbµÄĆū³Ę·Ö±šŹĒ”” ””ŗĶ”” ””£®
¢ŚÖ¤Ć÷“ęŌŚCO2µÄĻÖĻóŹĒ”” ””£¬Ö¤Ć÷“ęŌŚCOµÄĻÖĻóŹĒ”” ””£¬DÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ”” ””£®
¢Ū×°ÖĆAµÄ×÷ÓĆŹĒ”” ””£¬ĘųÄŅµÄ×÷ÓĆŹĒ”” ””£®
£Ø3£©ĪŖ²ā¶Øѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮ½ÖÖČēĻĀ·½°ø£®
| ČŪµć | ·Šµć | ČČĪČ¶ØŠŌ | Óė¼ī·“Ó¦ |
| 101”ćC”«102”ćC | 150”ćC”«160”ćCÉż»Ŗ | 100.1”ćCŹ§Č„½į¾§Ė®£¬175”ćC·Ö½ā³ÉCO2£¬CO£¬H2O | ÓėCa£ØOH£©2·“Ó¦²śÉś°×É«³Įµķ£ØCaC2O4£© |
¢Ł³ĘŅ»¶ØĮæѳʷÓĆÉĻĶ¼×°ÖĆ½ųŠŠŹµŃ飬²āµĆ×°ÖĆD·“Ó¦Ē°ŗóµÄÖŹĮæ²ī£¬ÓÉ“Ė¼ĘĖć³öµÄŹµŃé½į¹ū±ČŹµ¼ŹÖµĘ«µĶ£¬ÅųżŅĒĘ÷ŗĶ²Ł×÷µÄŅņĖŲ£¬ĘäŌŅņæÉÄÜÓŠ£ŗCOĪ“ĶźČ«·“Ó¦”¢”” ””£®
¢Ś³ĘČ”8.75g²ŻĖį¾§ĢåѳʷÅäÖĘ50.00gČÜŅŗ£¬Č”10.00gČÜŅŗ¼ÓŹŹĮæµÄĻ”ĮņĖį£¬Č»ŗóµĪ¼Ó25.00g3.16%KMnO4ČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«£®
£ØŅŃÖŖ£ŗ2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2”ü+8H2O£©ŌņKMnO4ČÜŅŗ
ĻŌ”” ””É«£¬25.00g3.16%KMnO4ČÜŅŗÖŠKMnO4µÄÖŹĮæ”” ””g£®Ēė¼ĘĖćѳʷ֊µÄÖŹĮæ·ÖŹż£®[Š“³ö¼ĘĖć¹ż³Ģ£¬M2£ØH2C2O4£©=90£¬M2£ØH2C2O4•2H2O£©=126£¬M2£ØKMnO4£©=158]£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³ŃŠ¾æŠ”×éÓū¼ģŃé²ŻĖį¾§Ģåѳʷ·Ö½ā²śĪļ£¬²¢²ā¶Øѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹż£Ø¼ŁÉčŌÓÖŹ²»²ĪÓė·“Ó¦£©”£ŅŃÖŖ£ŗÅØĮņĖįæÉ×÷ĪŖøÉŌļ¼Į£»²ŻĖį¾§Ģå£ØH2C2O4”¤2H2O £©µÄŠŌÖŹ¼ūĻĀ±ķ£ŗ
| ČŪµć | ·Šµć | ČČĪČ¶ØŠŌ | ĘäĖū |
| 101”ꔫ102”ę | 150”ꔫ160”ęÉż»Ŗ | 100.1”ę·Ö½ā³öĖ®£¬175”ę·Ö½ā³ÉCO2”¢CO”¢H2O | Óė Ca(OH)2·“Ó¦²śÉś°×É«³Įµķ(CaC2O4) |
£Ø1£©Ķ¼ 1 ŹĒ¼ÓČČ×°ÖĆ”£×īŹŹŅĖµÄ¼ÓČČ·Ö½ā²ŻĖį¾§Ģå×°ÖĆŹĒ c ”£ČōŃ”×°ÖĆ a æÉÄÜ»įŌģ³ÉµÄŗó¹ūŹĒ_____________________£»ČōŃ”×°ÖĆ b æÉÄÜ»įŌģ³ÉµÄŗó¹ūŹĒ_________________”£

£Ø2£©Ķ¼ 2 ŹĒŃéÖ¤ČČ·Ö½ā²śĪļÖŠŗ¬ CO ”¢ CO2µÄ×°ÖĆ”£

¢Ł ×°ÖĆ A µÄ×÷ÓĆŹĒ_____________________£¬ĘųÄŅµÄ×÷ÓĆŹĒ_____________________”£
¢Ś Ö¤Ć÷“ęŌŚ CO2µÄĻÖĻóŹĒ______________£¬Ö¤Ć÷“ęŌŚ CO µÄĻÖĻóŹĒ______________”£
£Ø3£©ĪŖ²ā¶Øѳʷ֊²ŻĖį¾§ĢåµÄÖŹĮæ·ÖŹż£¬Éč¼ĘČēĻĀ·½°ø£ŗ³ĘČ”Ņ»¶ØĮæѳʷ£¬ÓĆÉĻŹö×°ÖĆ½ųŠŠŹµŃ飬³ĘĮæ×°ÖĆ D ·“Ó¦Ē°ŗóµÄÖŹĮæ²ī”£ÓÉ“Ė¼ĘĖć³öµÄŹµŃé½į¹ū±ČŹµ¼ŹÖµĘ«µĶ£¬ÅųżŅĒĘ÷ŗĶ²Ł×÷ŅņĖŲ£¬ĘäæÉÄÜŌŅņ£ŗ_________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com