
| ÅØ¶ČŹ±¼ä£Ømin£©Ģõ¼ž | 30% H2O2 | 15% H2O2 | 5% H2O2 |
| ¼ÓČėag MnO2 | 0.2 | 0.8 | 2.0 |
| ¼ÓČėag Fe2O3 | 7.0 | 9.0 | 16.0 |


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢°ŃĻšĘ¤ČūČū½ųŹŌ¹ÜæŚŹ±£¬Ó¦°ŃŹŌ¹Ü·ÅŌŚ×ĄÉĻÓĆĮ¦Čū½ōĻšĘ¤Čū |
| B”¢½«½šŹōæÅĮ£·ÅČėŹŌ¹ÜŹ±£¬Ó¦ĻČ°ŃŹŌ¹Üŗį·Å£¬°Ń½šŹōæÅĮ£·ÅŌŚŹŌ¹ÜæŚ£¬ŌŁ°ŃŹŌ¹ÜĀżĀżŹśĮ¢ |
| C”¢ĻøæŚĘæµ¹ŅŗĢåŹ±£¬±źĒ©Ņ»ĆęŅŖÕż¶ŌŹÖŠÄ |
| D”¢¼ģ²éĘųĆÜŠŌŹ±£¬Ó¦ĻȰѵ¼¹ÜŅ»¶Ė·ÅČėĖ®ÖŠ£¬ŌŁÓĆŹÖĪÕ×”ŹŌ¹Ü£¬¹Ū²ģµ¼¹ÜæŚŹĒ·ńÓŠĘųÅŻ²śÉś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Ļ”ĮņĖį | B”¢Ļ”ŃĪĖį |
| C”¢ĮņĖįĆ¾ČÜŅŗ | D”¢ĮņĖįĶČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
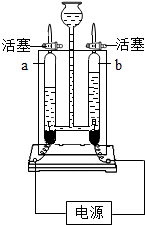 ČēĶ¼ŹĒµē½āĖ®µÄŹµŃé×°ÖĆ£¬ŅĄĶ¼»Ų“š£®
ČēĶ¼ŹĒµē½āĖ®µÄŹµŃé×°ÖĆ£¬ŅĄĶ¼»Ų“š£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Õż”¢øŗ¼«²śÉśµÄĘųĢåÖŹĮæ±ČŹĒ1£ŗ2 |
| B”¢Ö¤Ć÷ĮĖĖ®ŹĒÓÉĒāĘųŗĶŃõĘų×é³É |
| C”¢ÓėµēŌ“Õż¼«ĻąĮ¬µÄŅ»¶Ė²śÉśĒāĘų |
| D”¢Ė®Äܵē½āĖµĆ÷ĮĖ·Ö×ÓŹĒæÉ·ÖµÄ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| A”¢1£ŗ2£ŗ3 |
| B”¢1£ŗ2£ŗ6 |
| C”¢3£ŗ16£ŗ12 |
| D”¢3£ŗ16£ŗ24 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com