”¾“š°ø”æ
·ÖĪö£ŗ£Ø1£©øł¾ŻĢāŅā£¬“ÓCuSO
4+Fe=FeSO
4+Cu£¬æÉŅŌ擳öĢś×Ŗ»Æ³öĶ£¬ÕāŹĒŅ»øöŹ¹¹ĢĢåÖŹĮæŌö¼ÓµÄ¹ż³Ģ£¬¶ųĢāÄæĆ÷Č·µÄĖµĆ÷¹żĀĖŗóµÄ¹ĢĢåÖŹĮæÓėĶ¶ČėµÄĢś·ŪÖŹĮæĻąĶ¬£¬ÕāÖ»ÄÜĖµĆ÷Ńõ»ÆĶ±»Čܽāŗó£¬ĮņĖįČŌȻӊŹ£Óą£¬Ź£ÓąµÄĮņĖį¼ĢŠųĻūŗÄĢś·Ū£¬ĒŅĻūŗÄĢś·ŪµÄÖŹĮæÓėĒ°Ņ»²½¹ĢĢåŌö¼ÓµÄÖŹĮæĻąµČ£»ĄūÓĆÕāŅ»µČĮæ¹ŲĻµ£¬¼ĘĖćĖł¼ÓČėŃõ»ÆĶµÄÖŹĮ棻ÓÉĢāŅāæÉÖŖ£¬ĮņĖį×īÖÕČ«²æ×°»ÆĪŖĮņĖįŃĒĢś£¬øł¾ŻĮņĖįøłŹŲŗćæÉŅŌĒó³ö¼ÓČėĢś·ŪµÄÖŹĮæWµÄÖµ£»
£Ø2£©øł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬ÉÕ±¼°ĘäÖŠĪļÖŹ¼õÉŁµÄÖŹĮæ¾ĶŹĒÉś³ÉµÄ¶žŃõ»ÆĢ¼µÄÖŹĮ棬ÓɶžŃõ»ÆĢ¼µÄÖŹĮ棬øł¾ŻĢ¼ĖįøĘÓėŃĪĖį·“Ó¦µÄ·½³ĢŹ½æÉĒó³öĢ¼ĖįøĘ”¢ĀČ»ÆøʵÄÖŹĮ棬ÓÉŅŌÉĻŹż¾Ż¾ĶæÉŅŌ½ųŠŠÓŠ¹ŲµÄ¼ĘĖć£®
½ā“š£ŗ½ā£ŗ£Ø1£©ÉčŌĻČŃõ»ÆĶµÄÖŹĮæĪŖm£¬ŌņÓėŃõ»ÆĶ·“Ó¦µÄĮņĖįµÄÖŹĮæx£¬Éś³ÉĮņĖįĶµÄÖŹĮæy
CuO+H
2SO
4ØTCuSO
4+H
2O
80 98 160
m x y
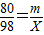
x=
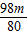
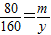
y=2m
Éč¼ÓČėĢś·ŪÓėĮņĖįĶČÜŅŗ³ä·Ö·“Ó¦¹ĢĢåŌö¼ÓµÄÖŹĮæĪŖa£»ÓėŹ£ÓąĻ”ĮņĖį·“Ó¦µÄĢśµÄÖŹĮæĪŖb£¬
Fe+CuSO
4ØTFeSO
4+Cu ¹ĢĢåÖŹĮæŌö¼Ó
56 160 64 64-56=8
2m a
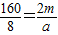
Fe+H
2SO
4ØTFeSO
4+H
2ӟ
56 98
b 100g×14%-

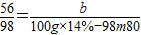
b=8-0.7m
·“Ó¦Ē°ŗó¹ĢĢåÖŹĮæ²»±ä£¬¼“ÓėĮņĖį·“Ó¦ĻūŗÄĢśµÄÖŹĮæµČÓŚÓėĮņĖįĶ·“Ó¦¹ĢĢåŌö¼ÓµÄÖŹĮ棬¼“£ŗa=b
0.1m=8-0.7m ½āµĆm=10g
ÓÉĢāŅāæÉÖŖ£¬ĮņĖį×īÖÕČ«²æ×°»ÆĪŖĮņĖįŃĒĢś£¬ÉčĮņĖįŃĒĢśµÄÖŹĮæĪŖZ£ŗ
H
2SO
4 ”«FeSO
4
98 152
14g×100% Z
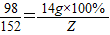
Z=
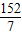
g
¼ÓČėĢś·ŪµÄÖŹĮæW=
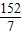
g×
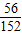
×100%=8g
£Ø2£©¢ŁÓÉĢāŅāæÉÖŖ£¬·“Ó¦ÖŠÉś³ÉµÄ¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ£ŗ
25g+115g+100g-231.2g=8.8g
Éč25gŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæĪŖp£¬·“Ӧɜ³ÉĀČ»ÆøʵÄÖŹĮæĪŖq
CaCO
3+2HClØTCaCl
2+H
2O+CO
2ӟ
100 111 44
p q 8.8g

½āµĆ£ŗp=20g
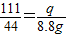
½āµĆ q=22.2g
ŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ·ÖŹżĪŖ

×100%=80.0%
¢Ś·“Ó¦ŗóČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ£ŗ
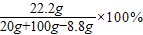
=20.0%
“š£ŗ£Ø1£©10£¬8£»£Ø2£©¢ŁŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ·ÖŹżŹĒ80.0%£¬¢Ś·“Ó¦ŗóČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżŹĒ20.0%
µćĘĄ£ŗ½ā“š£Ø1£©ĢāŹ±£¬·ÖĪö¹ĢĢåÖŹĮæ²»±äµÄŌŅņŹĒ½ā¾ö±¾ĢāµÄ¹Ų¼ü£ŗŅŖŹ¹¼ÓČėµÄĢś·ŪŗĶµĆµ½µÄ¹ĢĢåÖŹĮæĻąµČ±ŲŠėŹ¹ĮņĖįĻūŗĶąÓąµÄFe£¬ĒŅĻūŗÄĢś·ŪµÄÖŹĮæÓėÖĆ»»³öĶ¹ĢĢåŌö¼ÓµÄÖŹĮæĻąµČ£®

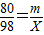 x=
x=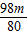
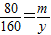 y=2m
y=2m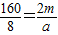

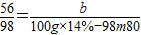 b=8-0.7m
b=8-0.7m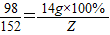 Z=
Z=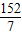 g
g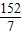 g×
g×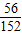 ×100%=8g
×100%=8g ½āµĆ£ŗp=20g
½āµĆ£ŗp=20g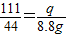 ½āµĆ q=22.2g
½āµĆ q=22.2g ×100%=80.0%
×100%=80.0% 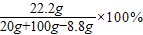 =20.0%
=20.0% 

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø