
���� ��1������Ӳˮ�����ķ��������ش�
��2��ϴ�ྫ���黯���ã�
��3������ά������������Ĺ�ϵ������
��4��������ͼ��ܷ����кͷ�Ӧ���з������
��5�����������Ҫ�ɷ�����ʯ�ң�CaO��������ˮ�ֺ�������������Ca��OH��2���ݴ�д����ѧ����ʽ��
��� �⣺��1���ճ������п���ͨ����еķ�������ˮ��Ӳ�ȣ�
��2����ϴ�ྫ��ϴ���ۣ���������ϴ�ྫ���黯���ã�
��3��ά���������������������³´�л��Ԥ���������ã�Ӧ��ʹ�ú�ά���طḻ�����ʣ�
��4��ʳ�׳����ԣ�������������ʷ�Ӧ��
��5����ʯ������ˮ���������������ƣ����Կ������������ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
�ʴ�Ϊ����1����У�
��2���黯��
��3��ά���أ�
��4��ʳ�ף�
��5��CaO+H2O=Ca��OH��2��
���� ��ѧ�����ǵ�����ϢϢ��أ���������������ص�֪ʶ���п�������ȵ�֮һ�����������ѧ֪ʶ����ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ����˳�� |
| ʾ������״���£��ܶ��ɴ�С | CO2��C2H4��C2H2��CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 7.2g | B�� | 6.0g | C�� | 9.6g | D�� | 8.0g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͭ���� | B�� | ��Ȼ����ú | C�� | �ƾ���ʯī | D�� | ʯ��ʯ����ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯ����ˮ��Ӧ | B�� | ��̿�������̼���·�Ӧ | ||
| C�� | þ���������� | D�� | Ũ��������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
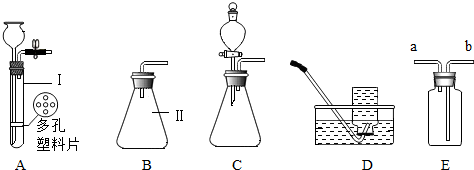
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 Ҫʹ��ͼװ���е�С�������Թ���������ʹ�õĹ����Һ������ǣ�������
Ҫʹ��ͼװ���е�С�������Թ���������ʹ�õĹ����Һ������ǣ�������| A�� | �٢� | B�� | �٢ڢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com