
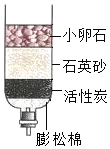
 Ė®ŹĒ×īĘÕĶØ”¢×ī³£¼ūµÄĪļÖŹÖ®Ņ»£®
Ė®ŹĒ×īĘÕĶØ”¢×ī³£¼ūµÄĪļÖŹÖ®Ņ»£®·ÖĪö £Ø1£©Š”ĀŃŹÆµÄ×÷ÓĆŹĒ¹żĀĖĘäÖŠµÄijŠ©“óµÄ²»ČÜŠŌŌÓÖŹ£¬Ķعż“Ė×°ÖĆ¾»»ÆŗóµĆµ½µÄĖ®ÖŠČŌČ»ŗ¬ÓŠŅ»Š©ČÜÓŚĖ®µÄĪļÖŹ£»
£Ø2£©øł¾Żµē½āĖ®ŹµŃéµÄ·“Ó¦ŌĄķ·ÖĪö½ā“š£»
£Ø3£©øł¾ŻÓ²Ė®Óė·ŹŌķĖ®»ģŗĻ²śÉś“óĮæµÄø”Ōü£¬ČķĖ®Óė·ŹŌķĖ®»ģŗĻ²śÉś½Ļ¶ąµÄÅŻÄ½ā“š£®
½ā“š ½ā£ŗ£Ø1£©½«»ė×ĒµÄŗÓĖ®ÓĆČēĶ¼ĖłŹ¾µÄ¼ņŅ×¾»Ė®Ę÷½ųŠŠ¾»»Æ£¬ĘäÖŠĘäÖŠŠ”ĀŃŹÆ”¢ŹÆӢɰµÄ×÷ÓĆŹĒ¹żĀĖĘäÖŠµÄijŠ©²»ČÜŠŌŌÓÖŹ£¬ÓĆ“Ė×°ÖĆ¾»»ÆŗóµĆµ½µÄĖ®ČŌČ»ŗ¬ÓŠŅ»Š©æÉČÜŠŌĪļÖŹ£¬ŹōÓŚ»ģŗĻĪļ£»
£Ø2£©µē½āĖ®Éś³ÉĒāĘųŗĶŃõĘų£¬ŹµŃéæÉÖ¤Ć÷Ė®ÓÉĒā”¢ŃõĮ½ÖÖŌŖĖŲ×é³É£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü£»
£Ø3£©ĻņĖ®ÖŠ¼ÓČė·ŹŌķĖ®Ź±£¬Čē¹ū²śÉś“óĮæµÄÅŻÄ£¬ĖµĆ÷ŹĒČķĖ®£»Čē¹ū²śÉśµÄÅŻÄŗÜÉŁ»ņ²»²śÉśÅŻÄ£¬ĖµĆ÷ŹĒÓ²Ė®£®×ŌĄ“Ė®Ļ“ŅĀ·žŹ±£¬·ŹŌķ²»Ņ×ĘšÅŻÄĒŅ²śÉśø”Ōü£¬ĖµĆ÷øĆ×ŌĄ“Ė®ŹĒÓ²Ė®£®
“š°ø£ŗ£Ø1£©¹żĀĖ£» »ģŗĻĪļ£»£Ø2£©2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü£»£Ø3£©Ó²Ė®£»
µćĘĄ ±¾ĢāÖ÷ŅŖ漲龻»ÆĖ®µÄ·½·Ø£¬½ā“šŹ±ŅŖ³ä·ÖĄķ½ā½ŚŌ¼ÓĆĖ®µÄŅāŅ壮



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | H2O2 | B£® | H2O | C£® | CH4 | D£® | H2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 Ķ¼¼×ĪŖX”¢Y”¢ZČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ¬øł¾ŻĒśĻßæÉÖŖ30oCŹ±XµÄČܽā¶ČĪŖ20g£®ŌŚ40oCŹ±£¬Š”Ć÷Ķ¬Ń§ÓūÅäÖĘ100æĖ28%µÄYĪļÖŹµÄČÜŅŗ£¬ĖūÄÜ£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©“ļµ½ÄæµÄ£®
Ķ¼¼×ĪŖX”¢Y”¢ZČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ¬øł¾ŻĒśĻßæÉÖŖ30oCŹ±XµÄČܽā¶ČĪŖ20g£®ŌŚ40oCŹ±£¬Š”Ć÷Ķ¬Ń§ÓūÅäÖĘ100æĖ28%µÄYĪļÖŹµÄČÜŅŗ£¬ĖūÄÜ£ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©“ļµ½ÄæµÄ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
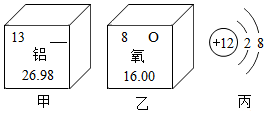 ČēĶ¼ĖłŹ¾ĪŖijŠ©ŌŖĖŲŗĶŌ×Ó½į¹¹µÄ²æ·ÖŠÅĻ¢£®
ČēĶ¼ĖłŹ¾ĪŖijŠ©ŌŖĖŲŗĶŌ×Ó½į¹¹µÄ²æ·ÖŠÅĻ¢£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·Ö×ÓµÄÖŹĮæ±äŠ” | B£® | ·Ö×Ó¼äÓŠ¼äøō | C£® | ·Ö×ÓŌŚ²»¶ĻŌĖ¶Æ | D£® | ·Ö×ÓÓÉŌ×Ó¹¹³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Cl | B£® | Cu | C£® | H2O2 | D£® | NaCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com