



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����ϡ����������g�� | 200 | 200 | 200 | 200 |
| ʣ�����������g�� | 37.6 | 15.2 | 4 | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��Ӧʱ��/�� | 0 | 2 | 4 | 6 | 8 | 10 |
| �ձ�����ʢ��������/�� | 80.0 | 79.0 | 78.3 | 77.9 | 77.8 | 77.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
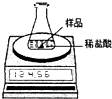 ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������
ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�飮ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������| ��Ӧǰ������װ��+ʯ��ʯ��Ʒ����/g | �����룺ϡ��������/g | ��Ӧ������װ��+��ƿ��ʣ���������/g |
| 104.60 | 20.00 | 123.72 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ���ݸ��������꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
ij��ѧ��ȤС��Ϊ�˴��Բⶨһ��ʯ��ʯ��Ʒ��CaCO3��������������Ʋ���������ʵ�顣
ʵ��װ����ͼ��ʾ����ȡ��ϸ��2.60gʯ��ʯ��Ʒ����4�μ���ϡ���ᣬ��ַ�Ӧ�����ٲ�������Ϊֹ����÷�Ӧǰ����й����������
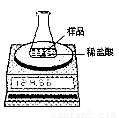

��1������ʯ��ʯ��Ʒ��CaCO3������������
��2����������ʵ�������ʯ��ʯ��Ʒ��������Һ��������������ͼ��װ���жϣ���ʵ��������??? ������ƫ��������ƫС������ȷ������ԭ����??? ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com