
| �ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ��Ʒ������g�� | 100 | 100 | 100 | 100 |
| ����������Һ������g�� | 40 | 80 | 120 | 160 |
| ����������g�� | 2.9 | 5.8 | 8.7 | 8.7 |
���� ��Ʒ����������������Һǡ����ȫ��Ӧʱ��Һ���Ȼ��Ƶ����������������Ȼ��Ƶ������ͷ�Ӧ���ɵ��Ȼ��Ƶ����������ݲ�������������þ���������Լ����Ȼ�þ�������������Ȼ�þ���������Լ����Ȼ��Ƶ���������һ�����Լ�����εĴ��ȣ�
��� �⣺��1���ɱ������ݿ�֪��������������Һ�����ӵ�120gʱ�������������������ӣ�˵����Ʒ���Ȼ�þȫ����Ӧ������ÿ����40g����������Һ�������ɳ���2.9g��������������Һ����Ϊ120gʱ�����ɳ���8.7g������������Һǡ����ȫ��Ӧ�����Ե����μ��������������Һ������е�MgCl2ǡ����ȫ��Ӧ��
��2���⣺����Ʒ��MgCl2������Ϊx
MgCl2+2NaOH�T2NaCl+Mg��OH��2��
95 58
x 8.7g
$\frac{95}{58}$=$\frac{x}{8.7g}$
x=14.25g
�������$\frac{100g-14.25g}{100g}$��100%�T85.75%
�ʴ�Ϊ��
��1����
��2��85.75%
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������һ����Ҫ�Ļ�������ԭ�ϣ���ҵ�����������Ậ�������Ȼ������ʣ�Ϊ�ⶨ��ҵ������̼���Ƶ�����������ijѧ���������ͼ��ʾ��ʵ��װ�ã�
������һ����Ҫ�Ļ�������ԭ�ϣ���ҵ�����������Ậ�������Ȼ������ʣ�Ϊ�ⶨ��ҵ������̼���Ƶ�����������ijѧ���������ͼ��ʾ��ʵ��װ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��һ�� | �ڶ��� | |
| ����ϡ��������� | mg | mg |
| �μ�����������Һ������ | 60g | 100g |
| �μ�����ͭ��Һ������ | 40g | 40g |
| ʵ������ | ���������� | ������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ᳫ����ĭ��ͺ� | |
| B�� | Ϊ�˷�ֹ����䣬�Դ����ļӵ��� | |
| C�� | ��������հ�װ���ٶ���ط�ֹ���ʸ��ñ��� | |
| D�� | ��������δ���е��ʵ�����������ĵ�����ˮ�ܵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 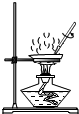 ���� | B�� |  ȡ�ù����ĩ | C�� |  ȡ��Һ�� | D�� |  ��˿��O2��ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡ����ʱ�����÷���װ�ú����������� | |
| B�� | û�и�ʴ�ԵĹ���ҩƷֱ�ӷ��������ϳ��� | |
| C�� | �ⶨij���Լ���pHʱ������ֽ�������Һ�� | |
| D�� | ��ʵ����ʣ��ҩƷ��Ҫ��ʱ�Ż�ԭ�Լ�ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
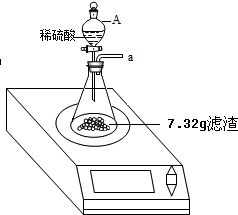 ij��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��
ij��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������������ | B�� | �ų��������� | ||
| C�� | ������ɫ���� | D�� | �������ݼ�����ζ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com