2008��9��27�գ�̫�յ�һ�������ˡ��й��˵Ľ�ӡ�����ҹ����Ƶĺ���Ա�����Ϊ����Ա�ɹ�����̫�������ṩ�˿ɿ��ı�֤��
��1�������ߡ����������족�������������ɣ����ڵ�������Ϊ������������紦����������ɵ����ʲ㡢�ϳ����ʵصı������ܲ㡢���Ϲؽڽṹ��ɵ������ܲ㡢�������ϵ����Ʋ㡢���Ȳ����������㣮��������Ȼ�л��߷��Ӳ�����ɵIJ���
��
A�����ʲ㣻B���������ܲ㣻C�������ܲ㣻D�����Ʋ�
��2������Ա������з�������ϵͳ���������������������裺
��һ�����÷�������һ��װ��ľ̿�ĺ��ӳ�ȥ��������һ����������ľ̿��
�ԣ�
�ʶ���������������ﮣ�LiOH�������ռ���ȥ������̼��������﮺��������ƶ��Ǽ�������ƵĻ�ѧ���ʣ���д������������ն�����̼�Ļ�ѧ����ʽ
��
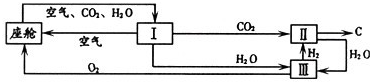
��3����������������ڿ������¹�������ͼ��ʾ��
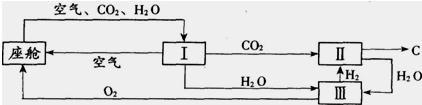
����װ��I�������Ƿ��������ˮ�Ͷ�����̼
��װ�â���CO
2��H
2�ķ�Ӧװ�ã��÷�Ӧ�Ļ�ѧ����ʽΪ
���ɲ�д����Ӧ��������װ�â�����Ӧ�Ļ�ѧ����ʽΪ
��
�ڴ�װ��I����ɿ�����O
2����Դ��CO
2��H
2O��������448 g O
2������506g CO
2����ͬʱ����H
2O
g��

 2008��9��27�գ�̫�յ�һ�������ˡ��й��˵Ľ�ӡ�����ҹ����Ƶĺ���Ա�����Ϊ����Ա�ɹ�����̫�������ṩ�˿ɿ��ı�֤��
2008��9��27�գ�̫�յ�һ�������ˡ��й��˵Ľ�ӡ�����ҹ����Ƶĺ���Ա�����Ϊ����Ա�ɹ�����̫�������ṩ�˿ɿ��ı�֤��