
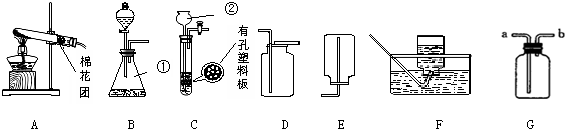
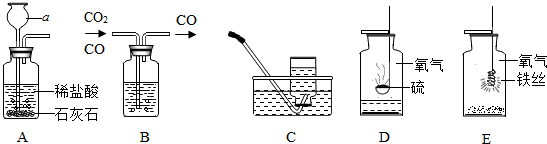
���� ��1���ݳ��������ش�
��2��ʵ���ҳ��ü��ȸ�����صķ�����ȡ���������ȼ�ղ����Ķ������������ж�����Ⱦ�������ݴ˷������
��3��Cװ�ÿ�ͨ������������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��4���ݷ�Ӧԭ����д����ʽ���������ܶ�С�ڿ���������Ӧ�Ӷ̹ܽ��������ܱ����ž�������
��� �⣺
��1�������������ƿ��
��2��ʵ��������Aװ����ȡ��������Ӧԭ���û�ѧ����ʽ��ʾΪ��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2�������ռ���������������ȼ��ʵ��ʱ��������ƿ�ڼ�����������������Һ�������������������ƿɷ�Ӧ�����Լ����������Ƶ�Ŀ���ǣ�����ȼ�����ɵĶ�������ֹ��Ⱦ������
��3����Cװ����ȡ������̼��ʹ�ø�װ����ȡ�����ͻ���ŵ��ǣ�����ͨ�������Ŀ�����ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��4��ʵ�����ü����Ȼ�狀��������ƹ�������ķ�����ȡ�����������Ȼ�狀��������ƹ�������ķ�����ȡ������ͬʱ�����Ȼ��ƺ�ˮ������ʽ�ǣ�2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2H2O+2NH3������ͼ����Gװ���ռ������������ܶȱȿ���С������Ӧ��b��ͨ�룻
�ʴ�Ϊ��
��1����ƿ��
��2��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2��������ȼ�����ɵĶ�������ֹ��Ⱦ������
��3��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��4��2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2H2O+2NH3����b��
���� ��ȷʵ������ȡ����װ��ѡȡ������������������ʣ����ܶ�װ�ý�����ȷ�����ۣ�


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuSO4��Һ | B�� | NaOH��Һ | C�� | ϡ���� | D�� | ZnSO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 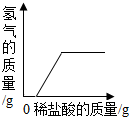 | B�� | 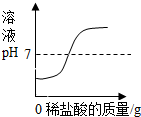 | C�� | 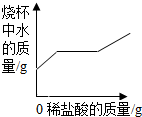 | D�� | 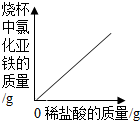 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
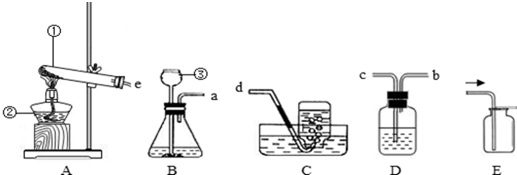
| ���� | ��ȡ�����ҩƷ | ��ȡ����ķ�Ӧ���� | ������������� |
| ���� | MnO2�����Ũ���� | ��Ҫ���� | ������ˮ���ܶȱȿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ú���ж���ָһ����̼�ж� | |
| B�� | �������϶����ںϳɲ��� | |
| C�� | ˮ���������������Ӫ����֮һ | |
| D�� | �ӵ�ʳ�ΰ�װ���ϵġ����������еĵ���ָ��Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ������ѡ�õ��Լ� | �������� | |
| A | KCl���壨KClO3�� | MnO2��H2O | ���Ⱥ��ˮ�ܽ���ˡ������ᾧ |
| B | H2��HCl�� | Na2CO3��Һ��ŨH2SO4 | ���ա����� |
| C | KNO3���壨KOH�� | H2O��CuSO4��Һ | �ܽ⡢���ˡ������ᾧ |
| D | CaCl2��Һ��HCl�� | ������CaO | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com