
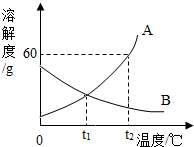
 ČēĶ¼ŹĒA”¢B¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻß
ČēĶ¼ŹĒA”¢B¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻß·ÖĪö £Ø1£©¾ŻøĆĪĀ¶ČĻĀAµÄČܽā¶Č·ÖĪö±„ŗĶČÜŅŗÖŠĖłŗ¬µÄČÜÖŹµÄÖŹĮ棻
£Ø2£©¾Ż¶žÕßµÄČܽā¶ČĖęĪĀ¶Č±ä»ÆĒéæö¼°±„ŗĶČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż¼ĘĖć·½·Ø½ā“š£»
£Ø3£©¾ŻABµÄČܽā¶ČĖęĪĀ¶Č±ä»ÆĒéæö·ÖĪö½ā“š£»
£Ø4£©²»±„ŗĶČÜŅŗ±äĪŖ±„ŗĶČÜŅŗæɲÉČ”½µĪĀµÄ·½·Ø£¬½įŗĻBµÄČܽā¶ČĖęĪĀ¶Č±ä»Æ·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©t2”ꏱAµÄČܽā¶ČŹĒ60g£¬¼“100gĖ®ÖŠ×ī¶ąČܽā60gµÄA£¬ŠĪ³É±„ŗĶČÜŅŗµÄÖŹĮæŹĒ160g£¬ĖłŅŌ½«t2”ꏱ32g AĪļÖŹµÄ±„ŗĶČÜŅŗÕōøÉ£¬ĄķĀŪÉĻæÉŅŌµĆµ½AĪļÖŹµÄÖŹĮæŹĒ $\frac{60g}{160g}$”Į32g=12g£»
£Ø2£©½«t2”ęĮ½ÖÖĪļÖŹµÄ±„ŗĶČÜŅŗ½µĪĀµ½t1”ę£¬AĪö³öČÜÖŹ£¬BČܽā¶Č±ä“ó£¬ČÜÖŹµÄÖŹĮæ·ÖŹżÓė½µĪĀĒ°ĻąµČ£¬±„ŗĶČÜŅŗÖŠČÜÖŹµÄČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż=$\frac{Čܽā¶Č}{Čܽā¶Č+100g}$”Į100%£¬¼“Čܽā¶ČŌ½“óÖŹĮæ·ÖŹżŅ²¾ĶŌ½“󣬶ųt1”ꏱAµÄČܽā¶Č“óÓŚt2”ꏱBµÄČܽā¶Č£¬ĖłŅŌAµÄÖŹĮæ·ÖŹż“óÓŚB£»ŹĒA “óÓŚB£»
£Ø3£©AµÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬BµÄČܽā¶ČĖęĪĀ¶ČÉżø߶ų¼õŠ”£¬ĖłŅŌČōAĪļÖŹÖŠ»ģÓŠÉŁĮæµÄBĪļÖŹ£¬æÉŅŌÓĆ ĄäČ“Čȱ„ŗĶČÜŅŗµÄ·½·Ø»ņ½µĪĀ½į¾§µÄ·½·ØĢį“æA£»
£Ø4£©BµÄČܽā¶ČĖęĪĀ¶ČÉżø߶ų¼õŠ”£¬ĖłŅŌBĪļÖŹµÄ²»±„ŗĶČÜŅŗ±äĪŖ±„ŗĶČÜŅŗ£¬æÉŅŌÓĆÉżøßĪĀ¶ČµÄ·½·Ø£»
¹Ź“š°øĪŖ£ŗ£Ø1£©12£»£Ø2£©“óÓŚ£» £Ø3£©ĄäČ“Čȱ„ŗĶČÜŅŗ£Ø»ņ½µĪĀ½į¾§£©£» £Ø4£©ÉżøßĪĀ¶Č£®
µćĘĄ Ć÷Č·Čܽā¶ČĒśĻßµÄŅāŅ唢Čܽā¶ČøÅÄī£¬¼°±„ŗĶČÜŅŗÖŠČܽā¶Č“óŌņČÜÖŹµÄÖŹĮæ·ÖŹż“ó”¢ĪļÖŹµÄ·ÖĄė”¢±„ŗĶČÜŅŗŗĶ²»±„ŗĶČÜŅŗµÄĻą»„×Ŗ»ÆµČÖŖŹ¶£¬¼“æɽįŗĻĢāŅāĖ³Ąū½ā“š£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
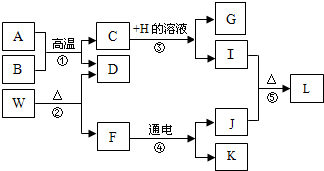 A”«L·Ö±šĪŖ¾ÅÄź¼¶»Æѧѧ¹żµÄ²»Ķ¬ĪļÖŹ£¬ĖüĆĒ“ęŌŚÓŅĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£®ŅŃÖŖAĪŖĢśŠāµÄÖ÷ŅŖ³É·Ö£¬B”¢D¾łĪŖĪŽÉ«ĘųĢ壬EŹÜČČŅ×·Ö½ā£¬FŌŚ³£ĪĀĻĀĪŖŅŗĢ壬HµÄČÜŅŗ³ŹĄ¶É«£®
A”«L·Ö±šĪŖ¾ÅÄź¼¶»Æѧѧ¹żµÄ²»Ķ¬ĪļÖŹ£¬ĖüĆĒ“ęŌŚÓŅĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£®ŅŃÖŖAĪŖĢśŠāµÄÖ÷ŅŖ³É·Ö£¬B”¢D¾łĪŖĪŽÉ«ĘųĢ壬EŹÜČČŅ×·Ö½ā£¬FŌŚ³£ĪĀĻĀĪŖŅŗĢ壬HµÄČÜŅŗ³ŹĄ¶É«£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ȱµā»įŅżĘšČ£³Ż | B£® | ȱĢś»įŅżĘšĘ¶ŃŖ | ||
| C£® | ȱøĘ»įŅżĘšĄĻÄźČĖ¹ĒÖŹŹčĖÉ | D£® | ȱŠæ»įŅżĘšŹ³Óū²»Õń£¬·¢Óż²»Į¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
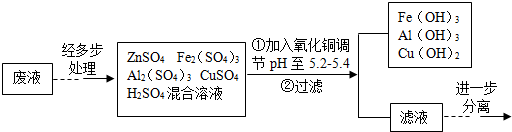
| Zn£ØOH£©2 | Fe£ØOH£©3 | Al£ØOH£©3 | Cu£ØOH£©2 | |
| æŖŹ¼³ĮµķµÄpH | 5.4 | 1.5 | 3.3 | 4.2 |
| ³ĮµķĶźČ«µÄpH | 8.0 | 3.7 | 5.2 | 6.7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ·“Ó¦Ē° | ·“Ó¦ŗó | ||
| ŹµŃé Źż¾Ż | ÉÕ±ŗĶĻ”ŃĪĖįµÄÖŹĮæ | ŹÆ»ŅŹÆѳʷµÄÖŹĮæ | ÉÕ±ŗĶĘäÖŠ»ģŗĻĪļµÄÖŹĮæ |
| 152.0g | 12.0g | 159.6g | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
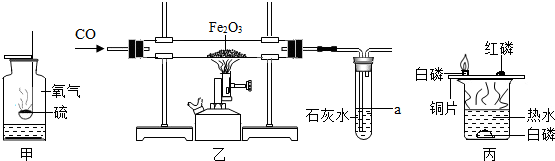
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com