
��7 �֣�����ͼ��ʾΪʵ�����г��������Ʊ�������������ռ�������ʵ��IJ���������ijѧУ������ѧʵ��̽���С���ͬѧ����������ɸ��Ե�̽��ʵ�顣 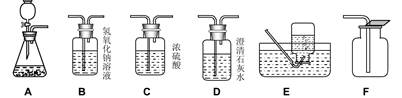
��1����һ���ͬѧ�Թ���������ҺΪԭ�ϣ�MnO2Ϊ������ ����ʵ�������Ʊ����ռ�һƿ���﴿��������������Ҫ�����ʵ��װ�� ������������������װ�õ������ԡ�
����ѡ����������˳��Ϊ �� ������д���������ĸ����
������ A ����������Ӧ�Ļ�ѧ����ʽΪ ��
����֤�����ռ����ķ����� ��
��2���ڶ����ͬѧΪ̽��������̼�����ʣ�����������ʵ�顣
�ټ�������ΪCO2Ӧѡ�����������е� ���B����D�� ���� �������Ļ�ѧ��Ӧ����ʽΪ ��
�ڽ�������̼ͨ������������Һ�У��������Է�Ӧ��������д��һ�ֻ�ѧ������֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ�������ǣ�д���Լ��������ۣ��� ��
��1����A��C��F ��2H2O2MnO2 2H2O+ O2��
�۽������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ����˵�� O2�Ѽ�����
��2�� D �� CO2 + Ca(OH)2 = CaCO3��+ H2O
�� ȡ��Ӧ�����Һ�������Թ��У� ��������ϡ���ᣬ ������ð���� ֤�� CO2�� NaOH
��Һȷʵ�����˻�ѧ��Ӧ
���������������1�����Թ���������ҺΪԭ�ϣ�MnO2Ϊ����������ʵ�������Ʊ����ռ�һƿ���﴿����������������ѡ����������˳��ΪA��C��F
������ A ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2MnO2 2H2O+ O2��
����֤�����ռ����ķ����ǣ��������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ����˵�� O2�Ѽ���
��2���ټ�������ΪCO2��Ӧ������ͨ�����ʯ��ˮ�У�Ӧѡ�����������е�D���������Ļ�ѧ��Ӧ����ʽΪ��CO2 + Ca(OH)2 = CaCO3��+ H2O
�ڣ�֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ��һ����ͨ�������Ƿ���̼�������ɣ����Է����ǣ�ȡ��Ӧ�����Һ�������Թ��У� ��������ϡ���ᣨ�������ƻ��Ȼ��ƣ��� ������ð�����а�ɫ�������ɣ��� ֤�� CO2�� NaOH ��Һȷʵ�����˻�ѧ��Ӧ
���㣺ʵ��װ�õ�ѡ���������֤��������������̼������������Һ��Ӧ��̽��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijѧ�������һ����ͼ��ʾ�ļ������巢��װ�á��Իش�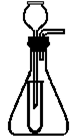
��1��װ����С�Թܵ������ǣ� ��
��2������װ�ÿ������Ʊ�ʲô���壿 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��9�֣���������ʵ��װ��ͼ���ش��й����⡣
��1��ָ������������ƣ��� ������ ��������
��2��ʵ��������Aװ�ÿ���ȡ��������Ӧ�Ļ�ѧ����ʽΪ����������������������������
��3��ʵ������ȡ���ռ�����Ӧѡ���װ������ ���� ������A~E��ѡ����Ӧ�Ļ�ѧ����ʽΪ�� ����ʹ�ø���װ����ȡ������ŵ����� �������� ��������Fװ���ռ�������������Ӧ���� �˽��롣
��4���ÿ�״ʯ��ʯ��ϡ���ᷴӦ���۲쵽�����ݳ������Ժ���Թ��ڲ���Һ��pH����ͼ��a����ʾ�������Һ������̼������Һ������pH����������õ���������ͼ����pHΪ�����꣬ʱ�䣨�룩Ϊ�����꣩����д��������bc�η�����Ӧ�Ļ�ѧ����ʽ���� ���������� ������ ����cd������ԭ������ ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣������Ǽ���ʵ������ȡ����ķ���װ�ú��ռ�װ�á�
��1��ʵ�����ø��������ȡ����ʱӦѡ�õķ���װ���� (����ĸ���)��
��2������װ���е�A��C��Ϻ�������ȡ���ռ��������� (��дһ��)��ʵ������ȡ������Ļ�ѧ����ʽ�� ��
��3��С����ʹ��ͼFװ���ռ��ϴ�������������Ӧ�Ƚ�װ����װ��ˮ���ٽ������ ���a����b������ͨ�롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(11��) ��������ʵ��װ��ͼ����Ҫ��ش��й����⣺
��1��д��ͼ�д��б�����������ƣ�a ��b ��
��2����ʵ���������������ȡ����ʱ���������Ļ�ѧ��Ӧ����ʽΪ ��
�ô����ǵ�ľ����������ʱ���ɹ۲쵽�������� ��
��3��ʵ������ȡ������̼���壬Ӧѡ��ķ���װ��Ϊ ����дװ�õ���ĸ���ţ���
ͬ����Ӧѡ�õ��ռ�װ���� �����ѡ��C�ռ����������̼��ˮ������Ӧ����
Ӧ�Ļ�ѧ����ʽΪ ��
��4�������dz��л�ѧ������Ҫ��ʵ���ʵ��װ�á�
��Aʵ�����ʵ������С��21%������ԭ���� ��д��һ�㣩��
��Bʵ���������Ϩ��˵��������̼���е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���ʵ������ȡ��������װ������ͼ��ʾ�� 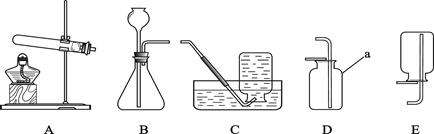
��ش��������⣺
��1��װ���бꡰa��������������_________________��
��2��ʵ�����ø��������ȡ������ѡ�õ��ռ�װ����_______������ĸ��ţ��� ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ�����ô���ʯ��ȡ������̼��ѡ�õķ���װ����_________����������Ϊ _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��9�֣����������װ�ã��ش����⣺
��1�� д����Ţ٢ڵ��������ƣ��� ���� ��
��2�� �ø��������ȡO2ʱ����A��D����ʵ�飬д���÷�Ӧ��ѧ����ʽ ������ˮ���е�ˮ����ԭ��Ϊ �����ռ����ʱ��Ӧ�� �����ˮ��ȡ�������ܡ���Ϩ��ƾ��ơ�����
��3�� ijͬѧ��ʵ�����ƶ�����̼����ʱ��д���÷�Ӧ��ѧ����ʽ ������ķ���װ����B�Ľ�ΪF���������ĺô��� ��
��4�� ʵ�����ü��ȹ�����ˮ�����ƺͼ�ʯ�һ����ķ����Ƽ��顣��ʵ���ѡ�õķ���װ
���� ��������ռ�װ�ÿ�ѡ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�������ͼ�ش����⡣
��1��ʵ������a�������� ��
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽΪ �����õķ���װ���� ����װ��D�ռ�������ԭ���� ��
��3��ʵ������ȡ���ռ�������̼ѡ�õ�װ���� ����Ӧ�Ļ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���۲�����װ�ò��ش�������⣮
��1����������c ���������� ����
��2��A װ���з�����Ӧ�Ļ�ѧ����ʽ���� ����
��3�����ø��������ȡ�������仯ѧ����ʽΪ�� ��������Dװ���ռ������������ķ������� ����
��4��F װ�ô�a��b ��ijһ���ӿڽ��������Դ���D��E װ���ռ����壬���ҿ��Լ�������������е��ݳ�������B��Fװ������ȡ���ռ�������̼���壬����B װ�õ�Ӧ���� ����ѡ�a����b������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com