
| pH | Ca2+”¢Mg2+×ÜŹż | Ļø¾ś×ÜŹż |
| 6.5”«8.5 | £¼2.709”Į1021øö?L-1? | £¼100øö?mL-1? |




| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| pH | Ca2+”¢Mg2+×ÜŹż | Ļø¾ś×ÜŹż |
| 6.5”«8.5 | £¼2.709”Į1021øö?L-1? | £¼100øö?mL-1? |
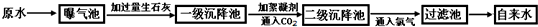
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌĖ®ŹĒČ”×ŌĢģČ»Ė®Ģå»ņŠīĖ®Ė®Ģ壬ČēŗÓĮ÷”¢ŗž²“”¢³ŲĢĮ»ņµŲĻĀŠīĖ®²ćµČ£¬ÓĆ×÷¹©Ė®Ė®Ō“µÄĖ®£¬Ķس£ŌĖ®ŗ¬ÓŠ½Ļ¶ąµÄæÉČÜŠŌøĘĆ¾»ÆŗĻĪļ£¬¶ųĪŅ¹śŅūÓĆĖ®ÖŹĮæ±ź×¼¹ę¶Ø±ŲŠė·ūŗĻĻĀ±ķÖŠŅŖĒó£ŗ
| pH | Ca2+ ”¢Mg2+×ÜŹż | Ļø¾ś×ÜŹż |
| 6.5”«8.5 | £¼ 2.709”Į1021øö·L-1 | £¼100øö·mL-1 |
ŅŌĻĀŹĒŌĖ®“¦Ąķ³É×ŌĄ“Ė®µÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼£ŗ
![]()
£Ø1£©Ä³×ŌĄ“Ė®³§ĖłČ”ŌĖ®ÖŠŗ¬½Ļ¶ąµÄCaCl2 ”¢MgCl2 ”¢Ca£ØHCO3£©2 µČ£¬¼ÓČė¹żĮæÉśŹÆ»ŅŹ±Ņ»¼¶³Į½µ³ŲÖŠ·¢ÉśĮĖČōøÉ»Æѧ·“Ó¦£¬ČēMg£ØHCO3£©2+Ca(OH)2=MgCO3”ż+CaCO3”ż+2H2OĒėŠ“³öĘäĖü»Æѧ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”££ØČĪŠ“1øö£©
£Ø2£©ŠõÄż¼ĮŌŚŌĖ®“¦ĄķĮ÷³ĢÖŠµÄ×÷ÓĆŹĒ£ŗ £»ĶØČėCO2µÄÄæµÄŹĒ ŗĶ ”£
£Ø3£©Š”![]() Ć÷Ķ¬Ń§²Ī¹ŪøĆ×ŌĄ“Ė®³§ŗó“ų»Ų¼ŅŅ»ĘæŌĖ®ŗĶŅ»Ęæ×ŌĄ“Ė®£¬“ŅƦ¼äĶü¼ĒĢłÉĻ±źĒ©£¬µ«Š”Ć÷ĄūÓĆ¼ŅÖŠ³£ÓĆĪļÖŹŗÜæģ½«Į½ĘæĖ®¼ų±š³öĄ“£¬ĖūŃ”ÓƵÄĪļÖŹŹĒ ”£
Ć÷Ķ¬Ń§²Ī¹ŪøĆ×ŌĄ“Ė®³§ŗó“ų»Ų¼ŅŅ»ĘæŌĖ®ŗĶŅ»Ęæ×ŌĄ“Ė®£¬“ŅƦ¼äĶü¼ĒĢłÉĻ±źĒ©£¬µ«Š”Ć÷ĄūÓĆ¼ŅÖŠ³£ÓĆĪļÖŹŗÜæģ½«Į½ĘæĖ®¼ų±š³öĄ“£¬ĖūŃ”ÓƵÄĪļÖŹŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌĖ®ŹĒČ”×ŌĢģČ»Ė®Ģå»ņŠīĖ®Ė®Ģ壬ČēŗÓĮ÷”¢ŗž²“”¢³ŲĢĮ»ņµŲĻĀŠīĖ®²ćµČ£¬ÓĆ×÷¹©Ė®Ė®Ō“µÄĖ®£¬Ķس£ŌĖ®ŗ¬ÓŠ½Ļ¶ąµÄæÉČÜŠŌøĘĆ¾»ÆŗĻĪļ£¬¶ųĪŅ¹śŅūÓĆĖ®ÖŹĮæ±ź×¼¹ę¶Ø±ŲŠė·ūŗĻĻĀ±ķÖŠŅŖĒó£ŗ
| pH | Ca2+ ”¢Mg2+×ÜŹż | Ļø¾ś×ÜŹż |
| 6.5”«8.5 | £¼ 2.709”Į1021øö·L-1 | £¼100øö·mL-1 |
ŅŌĻĀŹĒŌĖ®“¦Ąķ³É×ŌĄ“Ė®µÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼£ŗ
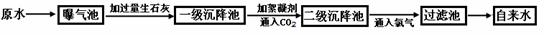
£Ø1£©Ä³×ŌĄ“Ė®³§ĖłČ”ŌĖ®ÖŠŗ¬½Ļ¶ąµÄCaCl2 ”¢MgCl2 ”¢Ca£ØHCO3£©2 µČ£¬¼ÓČė¹żĮæÉśŹÆ»ŅŹ±Ņ»¼¶³Į½µ³ŲÖŠ·¢ÉśĮĖČōøÉ»Æѧ·“Ó¦£¬ČēMg£ØHCO3£©2+Ca(OH)2=MgCO3”ż+CaCO3”ż+2H2OĒėŠ“³öĘäĖü»Æѧ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”££ØČĪŠ“1øö£©
£Ø2£©ŠõÄż¼ĮŌŚŌĖ®“¦ĄķĮ÷³ĢÖŠµÄ×÷ÓĆŹĒ£ŗ £»ĶØČėCO2µÄÄæµÄŹĒ ŗĶ ”£
£Ø3£©Š”Ć÷Ķ¬Ń§²Ī¹ŪøĆ×ŌĄ“Ė®³§ŗó“ų»Ų¼ŅŅ»ĘæŌĖ®ŗĶŅ»Ęæ×ŌĄ“Ė®£¬“ŅƦ¼äĶü¼ĒĢłÉĻ±źĒ©£¬µ«Š”Ć÷ĄūÓĆ¼ŅÖŠ³£ÓĆĪļÖŹŗÜæģ½«Į½ĘæĖ®¼ų±š³öĄ“£¬ĖūŃ”ÓƵÄĪļÖŹŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012Äź½ĖÕŹ”ĪŽĪżŹŠ±õŗžÖŠŃ§ÖŠæ¼»Æѧ¶žÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
| pH | Ca2+”¢Mg2+×ÜŹż | Ļø¾ś×ÜŹż |
| 6.5”«8.5 | £¼2.709×1021øö?L-1? | £¼100øö?mL-1? |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com