
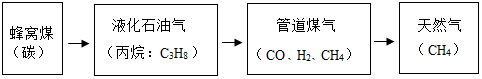
| ŹµŃé | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| Ļ”ŃĪĖįµÄÓĆĮæ | 5g | 5g | 5g | 5g |
| Ź£Óą¹ĢĢåµÄÖŹĮæ | X | 2g | 1g | 1g |
·ÖĪö £Ø1£©øł¾ŻµŚČż“Ī¼ÓČėŃĪĖįŗ󣬹ĢĢåÖŹĮæ¼õÉŁ1g£¬ĖłŅŌµŚŅ»“Ī¼ÓČėŃĪĖį¹ĢĢåÖŹĮæŅ²¼õÉŁ1g£¬ĖłŅŌmµÄÖµŹĒ4g-1g=3g½ųŠŠ·ÖĪö£»
£Ø2£©øł¾ŻŃłĘ·ÖŠ³żĢ¼ĖįøĘĶā£¬ĘäÓąµÄ³É·Ö¼Č²»ÓėŃĪĖį·“Ó¦£¬Ņ²²»ČÜÓŚĖ®£¬Ņņ“ĖµŚČż“Ī”¢µŚĖÄ“ĪŹµŃéĖłŹ£ÓąµÄ¹ĢĢ弓²»·¢Éś·“Ó¦Ņ²²»ČÜÓŚĖ®µÄŌÓÖŹ¹ĢĢåµÄÖŹĮæ½ųŠŠ·ÖĪö£»
£Ø3£©øł¾Ż»Æѧ·½³ĢŹ½ŗĶ²Ī¼Ó·“Ó¦µÄĢ¼ĖįøʵÄÖŹĮæ½ųŠŠ¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©µŚČż“Ī¼ÓČėŃĪĖįŗ󣬹ĢĢåÖŹĮæ¼õÉŁ1g£¬ĖłŅŌµŚŅ»“Ī¼ÓČėŃĪĖį¹ĢĢåÖŹĮæŅ²¼õÉŁ1g£¬ĖłŅŌmµÄÖµŹĒ4g-1g=3g£»
£Ø2£©øĆŹÆ»ŅŹÆѳʷ֊Ģ¼ĖįøʵÄÖŹĮæ·ÖŹżĪŖ£ŗ$\frac{4g-1g}{4g}$”Į100%=75%£»
£Ø3£©Éč²śÉś¶žŃõ»ÆĢ¼ĘųĢåµÄÖŹĮæĪŖx
CaCO3+2HClØTCaCl2+H2O+CO2”ü
100 44
3g x
$\frac{100}{3g}$=$\frac{44}{x}$
x=1.32g
“š£ŗŹµŃé¹ż³ĢÖŠ²śÉś¶žŃõ»ÆĢ¼ĘųĢåµÄ×ÜÖŹĮæĪŖ1.32g£®
¹Ź“š°øĪŖ£ŗ£Ø1£©3£»
£Ø2£©75%£»
£Ø3£©ŹµŃé¹ż³ĢÖŠ²śÉś¶žŃõ»ÆĢ¼ĘųĢåµÄ×ÜÖŹĮæĪŖ1.32g£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖ»Æѧ·½³ĢŹ½µÄ¼ĘĖć£¬ÄŃ¶Č²»“ó£¬×¢Ņā½āĢāµÄ¹ę·¶ŠŌŗĶ×¼Č·ŠŌ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĄÆÖņČŪ»Æ | B£® | ĘūÓĶ»Ó·¢ | C£® | Ö½ÕÅČ¼ÉÕ | D£® | ŗ£Ė®É¹ŃĪ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | -1 | B£® | +1 | C£® | +5 | D£® | +7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ČēĶ¼ĖłŹ¾¶¼ŹĒ³£¼ūµÄČÕ³£Éś»īÓĆĘ·£¬ĘäÖŠŹĒÓŠ»śøß·Ö×Ó²ÄĮĻµÄÓŠ£ŗ¢Ū¢Ž£ØĢīŠņŗÅ£©£®Éś»īÖŠŅŖ¼ų±šŃņĆ«ÉĄŗĶŗĻ³ÉĻĖĪ¬£¬æÉĶعżČ¼Éշصķ½·Ø£®
ČēĶ¼ĖłŹ¾¶¼ŹĒ³£¼ūµÄČÕ³£Éś»īÓĆĘ·£¬ĘäÖŠŹĒÓŠ»śøß·Ö×Ó²ÄĮĻµÄÓŠ£ŗ¢Ū¢Ž£ØĢīŠņŗÅ£©£®Éś»īÖŠŅŖ¼ų±šŃņĆ«ÉĄŗĶŗĻ³ÉĻĖĪ¬£¬æÉĶعżČ¼Éշصķ½·Ø£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
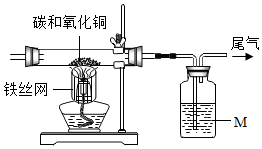 ѧĻ°Ģ¼µ„ÖŹµÄŠŌÖŹŹ±£¬Ä³Š£ŹµŃ銔×éµÄĶ¬Ń§ĪŖĢ½¾æĢ¼µÄ»ÆѧŠŌÖŹ£¬°“ĻĀĆęµÄŹµŃé×°ÖƶŌľĢæÓėŃõ»ÆĶµÄ·“Ó¦½ųŠŠĮĖŹµŃéĢ½¾æ£®ĒėÄćĶź³ÉĻĀĮŠĪŹĢā£®
ѧĻ°Ģ¼µ„ÖŹµÄŠŌÖŹŹ±£¬Ä³Š£ŹµŃ銔×éµÄĶ¬Ń§ĪŖĢ½¾æĢ¼µÄ»ÆѧŠŌÖŹ£¬°“ĻĀĆęµÄŹµŃé×°ÖƶŌľĢæÓėŃõ»ÆĶµÄ·“Ó¦½ųŠŠĮĖŹµŃéĢ½¾æ£®ĒėÄćĶź³ÉĻĀĮŠĪŹĢā£®| ŹµŃéĻÖĻó | ÓĆ»Æѧ·½³ĢŹ½½āŹĶ“ĖĻÖĻó |
| ¢ŁĮ§¹ÜÄŚŗŚÉ«·ŪÄ©Öš½„±äŗģ | C+2CuO$\frac{\underline{\;øßĪĀ\;}}{\;}$2Cu+CO2”ü |
| ¢Ś³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē | Ca£ØOH£©2+CO2ØTCaCO3”ż+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaOHŗĶHCl | B£® | Fe2O3ŗĶHCl | C£® | BaCl2ŗĶH2SO4 | D£® | NaOHŗĶCO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½šøÕŹÆ”¢ÉśĢś”¢»ĘĶ¶¼ŹōÓŚ½šŹō²ÄĮĻ | |
| B£® | ŗĻ³É²ÄĮĻ°üĄØŗĻ³ÉĻĖĪ¬”¢ŗĻ³ÉĻš½ŗ”¢ŗĻ½š | |
| C£® | æÉÓĆ³éĖ攢×ĘÉÕ”¢ĪÅĘųĪ¶µÄ·½·Ø¼ų±šŃņĆ«ĻĖĪ¬ŗĶĆŽĻĖĪ¬ | |
| D£® | ŹÆÄ«×öČ󻬼ĮŹĒŅņĪŖÖŹČķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com