
��֪ij����X�����������Ϣ����������Ҫ����Ļ�ѧ�ɷ���X2O3��������Ҫͨ���Ȼ�ԭ��ұ�����ɣ������������λ�ڽ���֮�ס�
(1)�ݴ��ƶ�X�������������� (��ѡ����ĸ)��
���� A���ѡ����� B���������� C���������� D��ͭ
(2)�ڸ�¯���ú�X2O3�Ŀ���ұ���ý�����ԭ������������������������������������
�������������������������������� (�û�ѧ����ʽ��ʾ)��
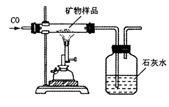
(3)ͬѧ�����������ʵ�鷽���ⶨ�ÿ�����X2O3����������(װ�����������ã������е����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ)��
��ȡ������Ʒ����������Ʒ��������
�ڲ����Ӧǰ���ƿ��ƿ��������������
�۲����Ӧ����ƿ��ƿ��������������
�ܼ���ó�������Ʒ��x2O3������������
����Ϊ����ʵ�鷽���������� (�һ������һ����)��![]() ȷ���������X2O3������
ȷ���������X2O3������
��������������������������������������
�������������������������������������������������������������������������� ��
���ı�װ�ú�ҩƷ���㻹����ͨ���ⶨ��Щ���ݣ���ͨ������ó�������X2O3������������
�������������������������������������������������������������������� ��������
���������������������������������������������������������������������������� ��
�ӻ����Ƕȿ�����װ�õIJ���֮������������������������������������������������ ��
 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
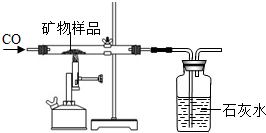 �����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ����
�����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
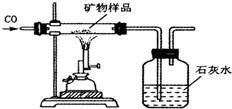 ��֪ij����X�����������Ϣ����������Ҫ����Ļ�ѧ�ɷ���X2O3��������Ҫͨ���Ȼ�ԭ��ұ�����ɣ������������λ�ڽ���֮�ף�
��֪ij����X�����������Ϣ����������Ҫ����Ļ�ѧ�ɷ���X2O3��������Ҫͨ���Ȼ�ԭ��ұ�����ɣ������������λ�ڽ���֮�ף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ��Ǩ����ԥʵ������п���ѧ��ģ�Ծ��������棩 ���ͣ������
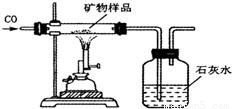
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com