
������������ѧ���ӻ�ѧ����������¿γ̸ĸ�����������ͨ����һ�껯ѧ��ѧϰ�������û�ѧ֪ʶ���������е�����������г��ֵ����⣺
��1��Ϊ����ʹ���ǰ��Ե���˳��ڱ��棬����һ���ǽ���˷����չ��У����ϸǺ����ڸǵ���Χ��������ˮ��������Ŀ���� �Է���˱��ʣ�
��2����¯��������Ҫԭ���У�����ʯ����̿��ʯ��ʯ�ȣ���¯�ڷ�������Ҫ��ѧ��Ӧ�У�
�ٽ�̿ȼ��Ϊ�����ṩ���� ��
���Խ�̿Ϊԭ����ȡ��ԭ�� ��
�۸������û�ԭ����ԭ����ʯ���Դ�����Ϊ����������� ��
��3��ij�ؾ����鷢���������д���ʹ�ú���ú��Ϊȼ����������������ŷŴ����Ķ�����̼���������꣬������������ĸĽ���ʩ������ú������������ʯ�Ҽ����������ɵĶ���������ԭ�����û�ѧ����ʽ��ʾΪ ��
��4��Ҫϴȥ��ˮ�õ������ڱ��ϵ�ˮ��[��Ҫ��CaCO3��Mg(OH)2]���ɼ�������ȥ����صĻ�ѧ����ʽΪ��CaCO3+2CH3COOH=(CH3COO)2Ca+H2O+CO2����Mg(OH)2+2CH3COOH=(CH3COO)2Mg+2H2O��������Ĵ���ܹ�������Ϊ�ᷢ����Ӧ___ ______________________________��ʹ������ʴ��
��1����������
��2��C+O2��ȼCO2 CO2+C����2CO Fe3O4+4CO����3Fe+4CO
��3��SO2+Ca(OH)2=CaSO3+H2O
��4��Fe+2CH3COOH=(CH3COO)2Fe+H2��
���������������1��ˮ�������ܷ�����ã������������룻��2��̼�������ڵ�ȼ���������ɶ�����̼��̼�������ڵ�ȼ����������һ����̼��һ����̼��������̼���ڸ����·�Ӧ�������Ͷ�����̼����3�������������������Ʒ�Ӧ����������ƺ�ˮ����4�����ڽ������˳������������ǰ�棬�ܽ���������û�������
���㣺ˮ����;��������ұ����ú����������ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��д����Ӧ�Ļ�ѧ�����ţ�����ʽ,��ע����Ӧ����.
����˿��������ȼ�գ� ����Ӧ���� .
��ͭ�����ȷֽ⣺ ����Ӧ���� .
��þ���ڿ�����ȼ�գ� ����Ӧ���� .
��̼��������ȷֽ⣺ . ��Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ش��������⣬д���йػ�ѧ����ʽ��
��1��������ͭ��Һ�м����Ȼ�����Һ���۲쵽�������� ����Ӧ�Ļ�ѧ����ʽΪ ��
��2��þ�ڿ�����ȼ����������þ�͵���þ�����е�Ϊ��3�ۣ�������þ��ˮ��Ӧ����������þ�Ͱ�����NH3����
��д������þ�Ļ�ѧʽ ��
����֪����þ��һ�ֻ���ɫ�Ĺ��壮����þ�ڿ�����ȼ�յ�ʵ������֪�������������£�þ����������е� ���ϣ������� ��
��д������þ��ˮ��Ӧ�Ļ�ѧ����ʽ ��
�� g����þ�к��� 24g þԪ�أ���ȷ�� 0.1g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���û�ѧ�����ʾ��
��3����ԭ�� ��
��2������������ ��
�ۿ����к����������� ��
��2Al+X��Al2O3+2Fe �У�X�Ļ�ѧʽ�� ��
��2��������Τ��һ�����͵Ŀ����в���ҩ���H7N9������һ����Ч��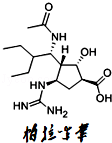
�ٴ�������Τ���ӵĻ�ѧʽ[C15H28N4O4]�У�������Щ��Ϣ�� ������д��һ���
������Ϊ������Τ�������ʵ��������������ڻ�ѧ���ʵ��� ������ţ���
| A���۵㣺130�� | B��������ΤΪ��ɫ��ĩ |
| C���ܶȣ�1.39g/cm3 | D���������Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѧϰ��������ἴ�̲ġ�����ѧ����ʳס�С�������Դ�����Ͽ�ѧ��ҽ�������ȷ���Խ��Խ��������Լ��ļ�ֵ��
��1��һ������ҩ������������ͭ��ȼ��ʱ������ɫ���棬��ѧ����ʽ���£�
2Cu��NO3��2 2CuO+O2+4X������X��һ�ֿ�����Ⱦ��仯ѧʽΪ ��
2CuO+O2+4X������X��һ�ֿ�����Ⱦ��仯ѧʽΪ ��
��2��������ԭ����һ����̼����������Ӧ��д���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��3��С����̽�����ƽ����������Ƿ��н���ͭ������ѡ�������Լ��е� ��
A��ZnC12��Һ B��Mg��NO3��2��Һ C��H2SO4��Һ D��AgNO3��Һ
��4��Ϊ̽��������ȼ�յ���������ͬʱ��ȼСľ����Сú�飬����Сú���ȼ����ʱ�䳤��ԭ���� ��
��5���������������pHΪ6���ҵ������У���ij������pHΪ4�������������ֲ��ȣ����������п����������������Ե��� ��
A����ʯ�� B����ʯ�� C��������� D���������ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�����ǻ�ѧѧϰ����Ҫ��ɲ��֣�������ȷ�Ļ�ѧ������գ�
��1��4�������� ��
��2������������Ԫ�صĻ��ϼ�Ϊ+1�� ��
��3����Է���������С�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�����dz��л�ѧѧϰ����Ҫ������
��1��д����Ԫ�ط��ŵ����壺
Fe��ʾ�� ����Fe����ʾ�� ����Fe���ɱ�ʾ�� ����
��2�����û�ѧ���ű�ʾ��
����������������� ������������ ��������۵���������Ļ�ѧʽ�� ��
�����á� ����ʾһ����ԭ�ӣ���
����ʾһ����ԭ�ӣ��� ����ʾ�������� ����
����ʾ�������� ����
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
�����ڿ�����ȼ���� ��
���ô���ʯ��ϡ������ȡ������̼�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���û�ѧ���Ż��������
��H�� ��
��Na+�� ��
��������� ��
����������
��2������������ ��
��2������Ԫ�ط�����ɽ������˳���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com