
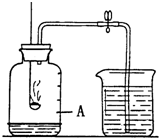
 æÕĘųŹĒŅ»ÖÖ±¦¹óµÄ×ŌȻ׏Ō“£®ČēĶ¼ĖłŹ¾×°ÖĆ£¬æÉŅŌ²ā¶ØæÕĘųĄļŃõĘųµÄŗ¬Į森ŹµŃ鏱ĻČŌŚ¼ÆĘųĘæĄļ¼ÓČėÉŁĮæĖ®£¬×öÉĻ¼ĒŗÅ£®ÓĆµÆ»É¼Š¼Š½ōČé½ŗ¹Ü£®µćČ¼Č¼ÉÕ³×ÄŚµÄŗģĮ×ŗó£¬Į¢¼“ÖŁČėĘæÖŠ²¢°ŃČū×ÓČū½ō£®“żŗģĮ×ĻØĆšŗ󣬷¢ĻÖŗģĮ×»¹ÓŠŹ£Óą£¬ĄäČ“£¬“ņæŖµÆ»É¼Š£¬ÉÕ±ÖŠµÄĖ®µ¹Į÷±Å¼ÆĘųĘæÖŠ£¬ŅŗĆęÉĻÉżµ½Ķ¼ÖŠAĪ»ÖĆ£®
æÕĘųŹĒŅ»ÖÖ±¦¹óµÄ×ŌȻ׏Ō“£®ČēĶ¼ĖłŹ¾×°ÖĆ£¬æÉŅŌ²ā¶ØæÕĘųĄļŃõĘųµÄŗ¬Į森ŹµŃ鏱ĻČŌŚ¼ÆĘųĘæĄļ¼ÓČėÉŁĮæĖ®£¬×öÉĻ¼ĒŗÅ£®ÓĆµÆ»É¼Š¼Š½ōČé½ŗ¹Ü£®µćČ¼Č¼ÉÕ³×ÄŚµÄŗģĮ×ŗó£¬Į¢¼“ÖŁČėĘæÖŠ²¢°ŃČū×ÓČū½ō£®“żŗģĮ×ĻØĆšŗ󣬷¢ĻÖŗģĮ×»¹ÓŠŹ£Óą£¬ĄäČ“£¬“ņæŖµÆ»É¼Š£¬ÉÕ±ÖŠµÄĖ®µ¹Į÷±Å¼ÆĘųĘæÖŠ£¬ŅŗĆęÉĻÉżµ½Ķ¼ÖŠAĪ»ÖĆ£®| 1 |
| 5 |
| 1 |
| 5 |
| 4 |
| 5 |
| 1 |
| 5 |
| 1 |
| 5 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃõĘų”¢Ė®ŗĶ¶žŃõ»ÆĢ¼ŹĒĪŅĆĒÉķ±ßÖŲŅŖµÄĪļÖŹ”£
””£Ø1£©A”¢B”¢C¶¼ŹĒŃŠ¾æĪļÖŹ×é³ÉŗĶŠŌÖŹµÄŹµŃ锣
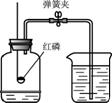
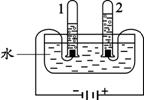

A.æÕĘųÖŠŃõĘųŗ¬Įæ²ā¶Ø B£®Ė®µÄµē½ā C£®Ė®µÄ¾»»Æ
¢Ł ¹ŲÓŚAĶ¼ĖłŹ¾ŹµŃ飬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ ”£
A£®ŹµŃ鏱ŗģĮ×Ó¦×ćĮæ B£®µćČ¼ŗģĮ×Ē°ĻČÓĆµÆ»É¼Š¼Š½ōČé½ŗ¹Ü
C£®ŗģĮ×ĻØĆšŗóĮ¢æĢ“ņæŖµÆ»É¼Š D£®×īÖÕ½ųČėĘæÖŠĖ®µÄĢå»żŌ¼ĪŖŃõĘųµÄĢå»ż
¢Ś BĶ¼ŹŌ¹Ü1ÖŠµÄĘųĢåĪŖ £»CĶ¼ÖŠ¾»»ÆĖ®µÄ·½·ØŹĒ ŗĶĪüø½”£
£Ø2£©ÓĆŹÆ»Ņ½¬ÄØĒ½£¬Ņ»¶ĪŹ±¼äŗóĒ½±Ś±äµĆÓÖ°×ÓÖÓ²£¬ŌŅņŹĒ £ØÓĆ»Æѧ·½³ĢŹ½»Ų“š£©”£
£Ø3£©¶žŃõ»ÆĢ¼ŹĒŅ»ÖÖ±¦¹óµÄ׏Ō“”£¹Ģ¶ØŗĶĄūÓƶžŃõ»ÆĢ¼µÄŅ»øö³É¹¦·¶ĄżŹĒ£ŗŌŚøßĪĀøßŃ¹ĻĀ¶žŃõ»ÆĢ¼ŗĶ°±Ęų£ØNH3£©æÉŅŌŗĻ³ÉÄņĖŲ[CO(NH2)2]£¬Ķ¬Ź±Éś³ÉĖ®”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©µĶĢ¼¾¼ĆŅŌµĶÄÜŗÄ”¢µĶÅÅ·Å”¢µĶĪŪČ¾ĪŖ»ł“”£¬Ę䏵֏ŹĒĢįøßÄÜŌ“ĄūÓĆŠ§ĀŹŗĶ““½ØĒå½ąÄÜŌ“½į¹¹”£ĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ ”£
A£®»šĮ¦·¢µēŹ±£¬½«Ćŗæé·ŪĖé³ÉĆŗ·ŪĄ“Ź¹ÓĆ
B£®½«Š£Ō°ĄļµÄĄ¬»ųĖĶµ½Ą¬»ų·ŁÉÕ·¢µē³§£¬·ŁÉÕ·¢µē
C£®ÓŻƽØÖžÉč¼Ę£¬ŌöĒæŹŅÄŚ×ŌČ»²É¹ā£¬¼õÉŁÕÕĆ÷ÓƵē
D£®ĄūÓĆĢ«ŃōÄܵČĒå½ąÄÜŌ““śĢę»ÆŹÆČ¼ĮĻ£¬ÓŠĄūÓŚ½ŚŌ¼ÄÜŌ“
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com