

 ×100%��
×100%��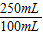 ×0.11g��0.28g��
×0.11g��0.28g��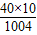 ×100%��39.8%��
×100%��39.8%��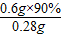 ��2�У�
��2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ԭ�Ӻ����
����ԭ�Ӻ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����ʽ�����ѧ������ѧ�����꼶���²� ���ͣ�038
���ԭ��������
H��1��C��12��O��16��P��31��Cl��35.5��Ca��40�����еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���
[Ca10(PO4)6(OH)2]�γɴ��ڣ�����Է�������Ϊ1004��ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺
(1)��װ��ǩ��֬����3.3g����ָ100mLţ���У���֬������������Ϊ3.3g����ôһ��ţ�̺�������________g��
(2)���ǻ�������и�Ԫ�ص�����������
(3)������ÿ��������Ҫ0.6g�ƣ�����Щ����90%����ţ�̣���һ����ÿ������Ҫ�ȶ��ٺ�ţ�̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������к����϶��Ԫ��֮һ������������ȱ�ƻ�����Ͳ��ͷ���������������ȱ�ƻᷢ���������ɣ������ۣ������еĸ�Ԫ����Ҫ���ǻ������[Ca10(PO4)6(OH)2]�������ʽ�����ڹ����������У����ж��ǻ�����Ƶ�˵������������
A���ǻ���������ڻ����
B���ǻ�����Ƶ�һ�������к���44��ԭ��
C���ǻ������������Ԫ��������Ca��P��O��HΪ200��93��208��l
D���ǻ����������Ԫ�صĻ��ϼ�Ϊ+5��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com