
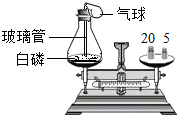
 £Ø2011?·šÉ½£©ĪŖĮĖŃŠ¾æÖŹĮæŹŲŗć¶ØĀÉ£¬Éč¼ĘĮĖÓŅĶ¼”°°×Į×Č¼ÉÕĒ°ŗóÖŹĮæ²ā¶Ø”±µÄŹµŃ飬Ēė·ÖĪöÓŠ¹ŲĪŹĢā£ŗ
£Ø2011?·šÉ½£©ĪŖĮĖŃŠ¾æÖŹĮæŹŲŗć¶ØĀÉ£¬Éč¼ĘĮĖÓŅĶ¼”°°×Į×Č¼ÉÕĒ°ŗóÖŹĮæ²ā¶Ø”±µÄŹµŃ飬Ēė·ÖĪöÓŠ¹ŲĪŹĢā£ŗ

 Ć¢¹ū½ĢøØŹī¼ŁĢģµŲÖŲĒģ³ö°ęÉēĻµĮŠ“š°ø
Ć¢¹ū½ĢøØŹī¼ŁĢģµŲÖŲĒģ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
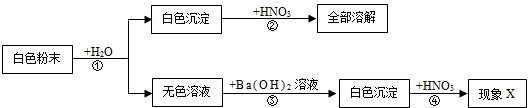
£Ø2011?·šÉ½£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬Ņ»øöĆܱÕČŻĘ÷ÄŚ·¢ÉśÄ³·“Ó¦£¬²āµĆ·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹµÄÖŹĮæ²æ·ÖŹż¾ŻČēĻĀ±ķĖłŹ¾£®ĻĀĮŠĪ“ÖŖŹż¾Ż¼ĘĖćÕżČ·µÄŹĒ£Ø””””£©
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com