
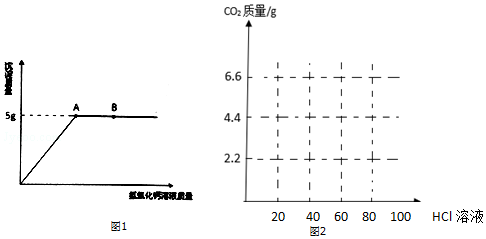
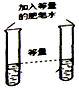
·ÖĪö £Ø1£©øł¾ŻBµćŹĒĒāŃõ»ÆøĘ¹żĮæµÄµć½ųŠŠ·ÖĪö£»
£Ø2£©øł¾Ż³ĮµķµÄÖŹĮæŗĶ»Æѧ·½³ĢŹ½½ųŠŠ¼ĘĖćĢ¼ĖįÄʵÄÖŹĮ棻
£Ø3£©øł¾ŻĒāŃõ»ÆÄĘÓėĢ¼ĖįÄʵÄÖŹĮæ±Č½įŗĻŃĪĖįµÄÖŹĮæ·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©¢ŁBµć¶ŌÓ¦µÄČÜŅŗŹĒĒāŃõ»ÆøĘŗĶĢ¼ĖįÄĘ·“Ó¦ŗóĒāŃõ»ÆøĘ¹żĮæµÄµć£¬Ņņ“ĖČÜŅŗÖŠµÄČÜÖŹÓŠ·“Ӧɜ³ÉµÄĒāŃõ»ÆÄĘŗĶ¹żĮæµÄĒāŃõ»ÆøĘ£»¹ŹĢī£ŗĒāŃõ»ÆÄĘŗĶĒāŃõ»ÆøĘ£»
¢ŚŹµŃ鶞֊ČōÓĆĀČ»ÆøĘČÜŅŗ“śĢęĒāŃõ»ÆøĘČÜŅŗ£¬ĖłµĆ³ĮµķĢ¼ĖįøʵÄÖŹĮæČ”¾öÓŚĢ¼Ėįøł½įŗĻøĘĄė×ÓµÄÖŹĮ棬ÓėŅõĄė×ÓĪŽ¹Ų£¬¹ŹÖŹĮæĻąµČ£»¹ŹĢī£ŗµČÓŚ£»
£Ø2£©Éčѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæĪŖx
Na2CO3+CaCl2=CaCO3”ż+2NaCl
106 100
x 5g
$\frac{106}{x}=\frac{100}{5g}$
x=5.3g
“š£ŗĢ¼ĖįÄʵÄÖŹĮæĪŖ5.3g£»
ѳʷµÄÖŹĮæĪŖ9.3g£¬¶ųĢ¼ĖįÄĘĪŖ5.3g£¬Ņņ“Ė»¹ŗ¬ÓŠĒāŃõ»ÆÄĘ£¬¹ŹŹĒ²æ·Ö±äÖŹ£»¹ŹĢī£ŗ²æ·Ö±äÖŹ£®
£Ø3£©ŃłĘ·µÄÖŹĮæĪŖ9.3g£¬¶ųĢ¼ĖįÄĘĪŖ5.3g£¬ŗ¬ÓŠĒāŃõ»ÆÄĘ9.3g-5.3g=4.0g
ÉčÓėĒāŃõ»ÆÄĘ·“Ó¦µÄŃĪĖįĪŖy£¬ÓėĢ¼ĖįÄĘ·“Ó¦µÄŃĪĖįĪŖz£¬Éś³É¶žŃõ»ÆĢ¼ĪŖn
Na2CO3+2HCl=2NaCl+H2O+CO2”ü£»NaOH+HCl=NaCl+H2O
106 73 44 80 36.5
5.3g z”Į7.3% n 4.0g y”Į7.3%
$\frac{106}{5.3g}=\frac{73}{z”Į7.3%}$ $\frac{106}{5.3g}=\frac{44}{n}$ $\frac{80}{4.0g}=\frac{36.5g}{y”Į7.3%}$
z=50g£¬y=50g£¬n=2.2g
ÓÉĶ¼ŃĪĖįÓÅĻČÓėĒāŃõ»ÆÄĘ·“Ó¦£¬ŗóÓėĢ¼ĖįÄĘ·“Ó¦£¬ĖłŅŌĶ¼Ź¾ĪŖ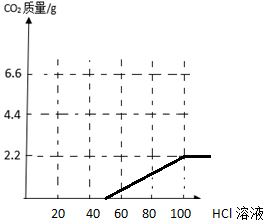 £®
£®
µćĘĄ “ĖĢāŹĒÓŠ¹ŲĒāŃõ»ÆÄʱäÖŹ³É·ÖµÄĢ½¾æĢā£¬ĘäÖŠÓÖÓŠøł¾Ż·½³ĢŹ½µÄ¼ĘĖćĢā£¬×ŪŗĻŠŌ½ĻĒ棬Äܹ»æ¼²éѧɜµÄ·ÖĪöĪŹĢāÄÜĮ¦£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2 | B£® | N2 | C£® | O2 | D£® | CO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²£Į§ĘĘĖé | B£® | Ę·ŗģĄ©É¢ | C£® | ĄÆÖņČ¼ÉÕ | D£® | øɱłÉż»Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆŹģŹÆ»ŅøÄĮ¼ĖįŠŌĶĮČĄ | |
| B£® | ½ÕøŃ”¢ŌÓ²Ż”¢·ą±ćµČŌŚÕÓĘų³ŲÖŠ·¢½ĶÖĘµĆ¼×Ķé | |
| C£® | ÓĆĒ¦”¢ī锢ĪżŗĶļÓÖĘ³ÉĪäµĀŗĻ½š | |
| D£® | ĀĢÉ«Ö²ĪļŅŌCO2ĪŖŌĮĻ£¬²śÉśO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 |  |  |  |
| A”¢ŃõĘųŌ¼Õ¼æÕĘųĢå»żµÄ$\frac{1}{5}$ | B”¢ĢśĖæÄÜŌŚŃõĘųÖŠČ¼ÉÕ | C”¢¢Ś¢Ū¶Ō±ČĖµĆ÷ĪļÖŹČ¼ÉÕŠčŅŖŃõĘų | D”¢¶žŃõ»ÆĢ¼µÄĆܶȱČæÕĘų“󣬲»Č¼ÉÕŅ²²»Ö§³ÖČ¼ÉÕ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢՓɲčŅ¶¹Ž | B£® | ²£Į§²čŅ¶¹Ž | C£® | ĀķæŚĢś²čŅ¶¹Ž | D£® | Ö½ÖĘ²čŅ¶¹Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ø”Ōüøü¶ąĪŖČķĖ®
ø”Ōüøü¶ąĪŖČķĖ® ĪĀ¶ČŌ½øߣ¬·Ö×ÓŌĖ¶ÆŌ½¾ēĮŅ
ĪĀ¶ČŌ½øߣ¬·Ö×ÓŌĖ¶ÆŌ½¾ēĮŅ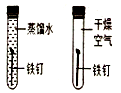 ĢśÉśŠāŠčŅŖŃõĘų
ĢśÉśŠāŠčŅŖŃõĘų ½šŹō»ī¶ÆŠŌÓÉĒæµ½ČõĢś”¢Ņų£®
½šŹō»ī¶ÆŠŌÓÉĒæµ½ČõĢś”¢Ņų£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2017½ģ¹ć¶«Ź”½ŅŃōŹŠ½ŅĪ÷ĻŲ¾ÅÄź¼¶ÖŠæ¼Ä£Äā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢī³äĢā
2016Äź”°ŹĄ½ē»·¾³ČÕ”±ÖŠ¹śČ·¶ØµÄÖ÷ĢāĪŖ”°øÄÉĘ»·¾³ÖŹĮ棬ĶʶÆĀĢÉ«·¢Õ¹”±”£ĻĀĮŠ×ö·ØÓėÖ®ĻąĪ„±³µÄŹĒ£Ø £©

A. Å©×÷ĪļµÄ½ÕøĖ¾ĶµŲ·ŁÉÕ
B. Éē»į¹«¹²³”ĖłŃĻ½ūĪüŃĢ
C. »ż¼«æŖÕ¹ĀĢ»Æ¹śĶĮŠŠ¶Æ
D. ¹¤Ņµ·ĻĖ®“¦Ąķ“ļ±źŗóÅÅ·Å
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com