
��֪ij������Ʒ�к���Na2SO4��MgCl2��CaCl2�����ʡ�ʵ�����ᴿ�������£�
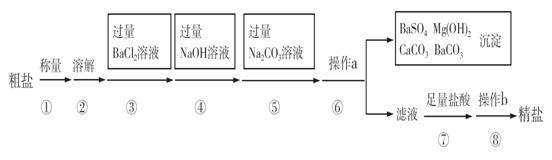
��1����������ƽ��������ʱ����ָ��ƫ���ұߣ����ʾ����������ȷѡ��Ĵ��룩 ��
A�������أ������� B�������ᣬ��Ʒ��
C�������أ���Ʒ�� D�������ᣬ������
��2���ڢܲ�����������Ӧ�Ļ�ѧ����ʽ�� ��
��3���ڢݲ�������Ŀ���� ��
��4���ڢ�����a�������� ���˲������У���������ĩ��Ҫ�����б����
��һ�ߡ�
��5���ڵڢ߲������У�����Һ�еμ����������Ŀ���� ��
��6���ڵڢಽ����ʱ��Ҫ�ò��������Ͻ��裬Ŀ���� ��
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Na+ | Mg2+ | Ca2+ | |
| OH- | �� | ���� | �� |
| Cl- | �� | �� | �� |
| CO32- | �� | �� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
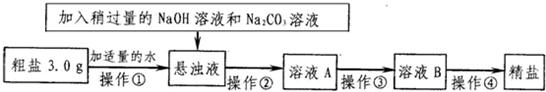
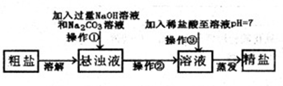 ��֪ij������Ʒ�к�������MgCl2��CaCl2�Ͳ��������ʣ�ͨ����ͼ��������ȡ���Σ�
��֪ij������Ʒ�к�������MgCl2��CaCl2�Ͳ��������ʣ�ͨ����ͼ��������ȡ���Σ�| Na+ | Mg2+ | Ca2+ | |
| OH- | �� | ���� | �� |
| Cl- | �� | �� | �� |
| CO32- | �� | �� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com