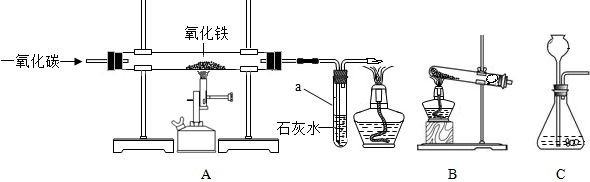
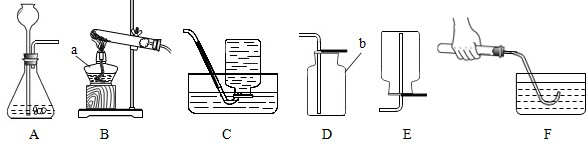
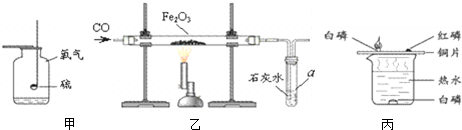
��2010?˳����һģ���������ͼ��ʾʵ��װ�ûش����⣮

��1��ͼ�б�������������Ǣ�
����©��
����©��
��
��2��Aװ�ÿ�������ȡ��������Ӧ�Ļ�ѧ����ʽΪ
���÷�����ȡ��������Ҫʵ�鲽���У��ټ��ȣ������Թ���װҩƷ���̶����ۼ��װ�������ԣ�������ˮ���ռ������� ��ֹͣ���ȣ������ܴ�ˮ����ȡ������ȷ��ʵ�����˳����_
�ۢڢ٢ܢޢ�
�ۢڢ٢ܢޢ�
������ţ���
��3��Bװ�ÿ�������ȡ���ռ�������̼����Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+CO2��+H2O
CaCO3+2HCl=CaCl2+CO2��+H2O
��ʵ�����������з�Ӧ��ȡ����Zn+H
2SO
4�TZnSO
4+H
2����
����
����
����ܡ����ܡ�����Bװ����ȡ���ռ�������
��4��Cʵ����˿�������о���ȼ�ա�
�������䣬�ų������ȣ����ɺ�ɫ���壻
�������䣬�ų������ȣ����ɺ�ɫ���壻
��ʵ��Ԥ���ڼ���ƿ�ڷ�������ˮ����������
��ֹƿ��ը��
��ֹƿ��ը��
��
��5��Dʵ������̽��ȼ�յ�������ʵ��ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա�
ˮ�а�����Ƭ�ϵİ���
ˮ�а�����Ƭ�ϵİ���
������õ��ģ�




 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�