
| 18.2g |
| 10% |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
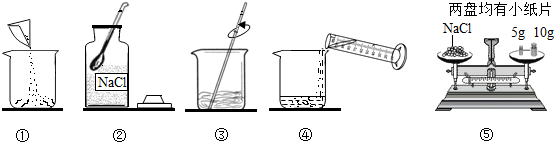
6�֣���ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
1.����ͼ��ʾ����ű�ʾ������Һ����ȷ˳��_____ ____��
2.ͼ���У���һ��������������������___ ______��
3.����NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ ��
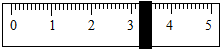
4.���ݼ�����Ҫ��ȡˮ������� ��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����_______����ѡ����ĸ��ţ�

5.����NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ�������������� ������ڡ�����С�ڡ����ڡ���10%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣���ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
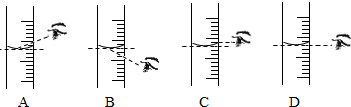
��1������ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��_______________________��
��2��ͼ���У���һ��������������������___________��
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ ��
��4�����ݼ�����Ҫ��ȡˮ������� ��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����_____����ѡ����ĸ��ţ�
��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ�������������� ������ڡ�����С�ڡ����ڡ���10%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ĵ�ʡ�ɶ����о��꼶3�·ݼ�⻯ѧ�Ծ����������� ���ͣ������
��6�֣���ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
��1������ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��_______________________��
��2��ͼ���У���һ��������������������___________��
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ���� ����ʾ������ͼ�����ȡ��NaCl����Ϊ ��
��4�����ݼ�����Ҫ��ȡˮ������� ��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����_____����ѡ����ĸ��ţ�
��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ�������������� ������ڡ�����С�ڡ����ڡ���10%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡӪ�����п�ģ�⣨������ѧ�Ծ� ���������� ���ͣ������
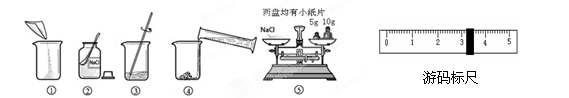
6�֣���ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
��С��1������ͼ��ʾ����ű�ʾ������Һ����ȷ˳��_____ ____��
��С��2��ͼ���У���һ��������������������___ ______��
��С��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ ��
��С��4�����ݼ�����Ҫ��ȡˮ������� ��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����_______����ѡ����ĸ��ţ�
��С��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ�������������� ������ڡ�����С�ڡ����ڡ���10%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ�ɽ�����վ�����ѧ ���ͣ������
��9�֣���ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��

��1������ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��_______________________��
��2��ͼ���У���һ��������������������___________��
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ ��

��4�����ݼ�����Ҫ��ȡˮ������� ��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����_____����ѡ����ĸ��ţ�
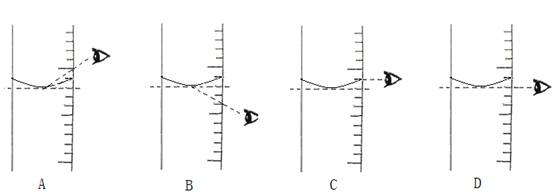
��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ�������������� ������ڡ�����С�ڡ����ڡ���10%��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com