
 �����벻����ѧ�������벻��ˮ��
�����벻����ѧ�������벻��ˮ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?�ɽ�����ģ�������벻����ѧ�������벻��ˮ��
��2013?�ɽ�����ģ�������벻����ѧ�������벻��ˮ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����벻����ѧ�������벻��ˮ��
���û�ѧʽ��գ�
����θҺ�к��е����� (1)  ��
��
�γ��������Ҫ������ (2) ��
�к��������Ե������� (3) ��
��ҽ���ϳ���75%�ľƾ���C2H5OH����Һ���� �������ƾ�Ħ�������� (4) ������̼���⡢��ԭ�����ʵ���֮��Ϊ (5) ��1.5molC2H5OH������Լ���� (6) ��̼ԭ�ӡ�
�������ƾ�Ħ�������� (4) ������̼���⡢��ԭ�����ʵ���֮��Ϊ (5) ��1.5molC2H5OH������Լ���� (6) ��̼ԭ�ӡ�
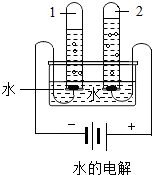
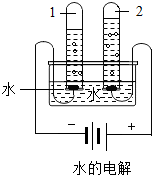
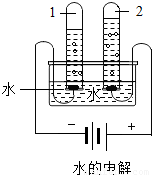
����ͼˮ���ʵ���У��Թ�2�еõ��������� (7) ������ˮ������ͨ�������������� (8) �����þ�ˮ����װ�������Ե������� (9) ��д�������ƣ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ϻ����ɽ����п���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com