
| Ļ”ŃĪĖįµÄÓĆĮæ | µŚ1“Ī¼ÓČė5g | µŚ2“Ī¼ÓČė5g | µŚ3“Ī¼ÓČė5g | µŚ4“Ī¼ÓČė5g |
| Ź£Óą¹ĢĢåµÄÖŹĮæ | 1.315g | 0.63g | 0.3g | 0.3g |
| 2g-0.3g |
| 2g |
| 100 |
| 2g”Į85% |
| 44 |
| x |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
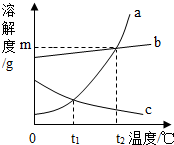
| A”¢0”ꏱ£¬Č”a”¢bø÷mg·Ö±š¼ÓČėµ½100gĖ®ÖŠ£¬ĪĀ¶ČÉżøßµ½t2”ꏱ£¬ĖłµĆČÜŅŗ¾ł±„ŗĶ |
| B”¢t1”ꏱ£¬a”¢cĪļÖŹµÄ±„ŗĶČÜŅŗÖŠ£¬ČÜÖŹµÄÖŹĮæĻąµČ |
| C”¢t2”ꏱ£¬½«a”¢bĮ½ĪļÖŹµÄ±„ŗĶČÜŅŗ£¬·Ö±šŗćĪĀÕō·¢µČÖŹĮæµÄĖ®Ź±£¬Īö³ö¾§ĢåµÄÖŹĮæŅ»¶ØĻąµČ£Ø¾§Ģå¾ł²»ŗ¬½į¾§Ė®£© |
| D”¢t1”ꏱ£¬a”¢cµÄ±„ŗĶČÜŅŗ“Ót1”ęÉżøßµ½t2”ꏱ£¬aĪļÖŹČÜŅŗČÜÖŹµÄÖŹĮæ·ÖŹż“óÓŚcĪļÖŹČÜŅŗČÜÖŹµÄÖŹĮæ·ÖŹż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 “ś±ķµŖŌ×Ó£¬
“ś±ķµŖŌ×Ó£¬ “ś±ķ ĒāŌ×Ó
“ś±ķ ĒāŌ×Ó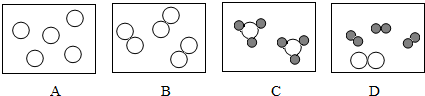
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
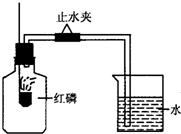 ŅŃÖŖæÕĘųµÄÖ÷ŅŖ³É·ÖŹĒµŖĘųŗĶŃõĘų£¬Ä³æĪĶā»ī¶ÆŠ”×éÉč¼Ę²ā¶ØæÕĘųÖŠŃõĘųŗ¬ĮæµÄŹµŃ飬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ
ŅŃÖŖæÕĘųµÄÖ÷ŅŖ³É·ÖŹĒµŖĘųŗĶŃõĘų£¬Ä³æĪĶā»ī¶ÆŠ”×éÉč¼Ę²ā¶ØæÕĘųÖŠŃõĘųŗ¬ĮæµÄŹµŃ飬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ| 1 |
| 5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢“Ó100g20%µÄijČÜŅŗÖŠČ”³ö50g£¬ĘäČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ10% |
| B”¢Ķł100g10%µÄijČÜŅŗÖŠ¼ÓČė10gĶ¬ÖÖČÜÖŹ£¬ĖłµĆČÜŅŗµÄČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ20% |
| C”¢°Ń100g10%µÄijČÜŅŗŗćĪĀÕō·¢45gĖ®£¬ĖłµĆČÜŅŗµÄČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ20% |
| D”¢ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬½«Ä³±„ŗĶČÜŅŗŗćĪĀÕō·¢Ņ»¶ØĮæĖ®£¬ĖüµÄČÜÖŹµÄÖŹĮæ·ÖŹż²»±ä |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ģ¼ŹĒČĖĄą½Ó“„×īŌē”¢ĄūÓĆ×ī¶ąµÄŌŖĖŲÖ®Ņ»£®
Ģ¼ŹĒČĖĄą½Ó“„×īŌē”¢ĄūÓĆ×ī¶ąµÄŌŖĖŲÖ®Ņ»£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
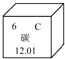
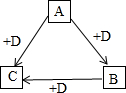 ČēĶ¼ÖŠµÄĪļÖŹ¾łŹĒ³õÖŠ»ÆѧµÄ³£¼ūĪļÖŹ£¬ĘäÖŠAĪŖŗŚÉ«¹ĢĢ壬B”¢C¾łĪŖĘųĢ壬ĒŅBÓŠ¾ē¶¾£¬Ēėøł¾ŻĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£ØÓŠŠ©·“Ó¦Ģõ¼žŅŃŹ”ĀŌ£©£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ÖŠµÄĪļÖŹ¾łŹĒ³õÖŠ»ÆѧµÄ³£¼ūĪļÖŹ£¬ĘäÖŠAĪŖŗŚÉ«¹ĢĢ壬B”¢C¾łĪŖĘųĢ壬ĒŅBÓŠ¾ē¶¾£¬Ēėøł¾ŻĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£ØÓŠŠ©·“Ó¦Ģõ¼žŅŃŹ”ĀŌ£©£¬»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com