
| ��Ӧǰ | ��Ӧ�� | |
| �ձ�������þ��Һ������ | ������������Ϊ10%��NaOH��Һ | ���˺��ձ�����Һ������ |
| 80g | 100g | 177.1g |
���� ��1�����������غ㶨�ɣ���Ӧǰ��������Ϊ��������������þ��������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ��������������þ���������������ĵ�����þ����������þ������������þ��Һ�������ȿɼ���MgSO4��Һ�����ʵ�����������
��3�����ݻ�������ijԪ�ص�����=�û��������������Ԫ�ص��������������з������
��� �⣺��1�����������غ㶨�ɣ���Ӧ����������þ����������Ϊ��80g+100g��-177.1g=2.9g�����2.9��
��2����MgSO4��Һ�����ʵ�����Ϊx
MgSO4+2NaOH�TNa2SO4+Mg��OH��2��
120 58
x 2.9g
$\frac{120}{x}=\frac{58}{2.9g}$
x=6g
MgSO4��Һ�����ʵ���������Ϊ$\frac{6g}{60g}$��100%=10%��
��MgSO4��Һ�����ʵ���������Ϊ10%��
��3����������=��Һ���������ʵ�����������100g������������Ϊ10%�����������ƣ�NaOH����Һ������Һ�����ʵ�����=100g��10%=10g���ܼ�����=��Һ����-����������������ˮ������=100g-10g=90g��
����Һ�к��е���Ԫ�ص�����Ϊ10g��$\frac{16}{23+16+1}$��100%+90g��$\frac{16}{1��2+16}$��100%=84g��
��100g������������Ϊ10%��NaOH��Һ����Ԫ�ص�����Ϊ84g��
���� ���⿼��ѧ��Ӧ��Ԫ���غ�ķ����������ǽ���еĹؼ���������������=��Һ���������ʵ������������ܼ�����=��Һ����-������������ѧʽ���йؼ��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ | B�� | ���սոѣ���Լ��Դ | ||
| C�� | ���������Դ | D�� | ��չ¶���տ����ḻ�Ϸ�С���Ļ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���еİ��Ǻ�ʳ�Ω�����ζ�� | B�� | ��ˮ��Ӳˮ��������ˮ | ||
| C�� | N2��CO2��������ζ | D�� | ��������������ȼ�ŵ�ľ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
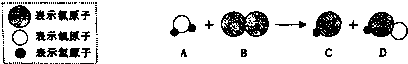
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ | B�� | ����� | C�� | ����� | D�� | ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaCl2 ��Һ | B�� | ʯ����Һ | C�� | AgNO3��Һ | D�� | ��̪��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KCl��K2CO3��������ϡ���ᡢ���ˡ��������ᾧ | |
| B�� | BaSO4��BaCO3��������ϡ���ᡢ���ˡ�ϴ�ӡ����� | |
| C�� | CuO��Cu��������ϡ���ᡢ���ˡ�ϴ�ӡ����� | |
| D�� | KCl ��MnO2�� ��ˮ�ܽ⡢���ˡ��������ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com