���𰸡�
��������һ�����ݷ��ӡ����Ӽ��۱���š���ѧʽ����д��������
������������д��ѧ����ʽ��ԭ��ѧʽ��ԭ����
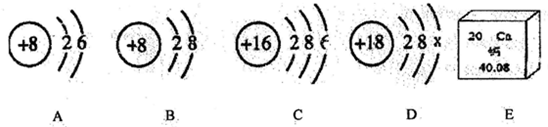
����⣺��һ����1�������к������������ǵ�������ѧʽΪ N
2��
��2�����ӵı�ʾ��������ȷ��д���ʵĻ�ѧʽ����ʾ����÷��ӣ������仯ѧʽǰ������Ӧ�����֣���˶���������ӱ�ʾΪ��2CH
4��
��3�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ���ʾ������������ӷ���ǰ������֣��������������ӷ���Ϊ 3Fe
2+��
��4��Ԫ�صĻ��ϼ۵���ȷ�귨����Ԫ�ط��Ż�ԭ���ŷ��ŵ����Ϸ��������ϼ۵�����ͼ�Ŀ��̼����̼Ԫ�صĻ��ϼ�Ϊ+4�ۣ�����Ϊ H
2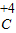
O
3��
��5������������õĽ�������������Ϊ Ag��
��6����K
2O��CaO��Na
20��MgO��x��ZnO�������ǰ��������˳������еģ����xΪ������������ΪAl
2O
3��������1���Ҵ���C
2H
5OH���ڿ����г��ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪ C
2H
5OH+3O
2 
2CO
2+3H
2O
��2���Ҵ����ڿ�������Դ����ͨ����ʳ������ȡ��A�����Ҵ����������Ҵ��м������ͣ��ǻ���B���� ʹ���Ҵ����Ϳɼ��ٴ�����Ⱦ��ȷ��ʹ���Ҵ����Ϳɽ�ʡʯ����Դ��ȷ����ѡAB��
��3��CO��NO�����ʹ��������·�����Ӧ���������ֳ�����������Ϊ������̼�͵�������ѧ����ʽΪ2CO+2NO

2CO
2+N
2�ʴ�Ϊ����һ����1��N
2����2��2CH
4����3��2Fe
2+����4��H
2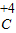
O
3����5��Ag����6��Al
2O
3��������1��C
2H
5OH+3O
2
2CO
2+3H
2O����2��AB ��3��2CO+2NO

2CO
2+N
2���������⿼�黯ѧ���ż���ѧ����ʽ����д�����ڻ���֪ʶ���飮

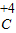 O3��
O3�� 2CO2+3H2O
2CO2+3H2O 2CO2+N2
2CO2+N2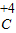 O3����5��Ag����6��Al2O3
O3����5��Ag����6��Al2O3 2CO2+3H2O����2��AB ��3��2CO+2NO
2CO2+3H2O����2��AB ��3��2CO+2NO 2CO2+N2
2CO2+N2


 26���������ͼ�ش�
26���������ͼ�ش�