
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ٳ�ȡ�����������ƹ�����Ʒ8.0g����50mLˮ�����Һ������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ���ã� | ������ɫ���� | ˵�����ù����У�һ������______���ѧʽ���� |
| ���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС���pH��ֽ�ϣ������ɫ���Աȣ����pH | pH=11 | ˵�����ù����У���һ������______���ѧʽ���� |
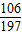 =
=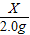
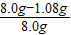 ×100%=86.5%
×100%=86.5%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�����������غ���Ƭ�п���ѧģ���Ծ��������棩 ���ͣ������
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ٳ�ȡ�����������ƹ�����Ʒ8.0g����50mLˮ�����Һ������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ���ã� | ������ɫ���� | ˵�����ù����У�һ������______���ѧʽ���� |
| ���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС��pH ��ֽ�ϣ������ɫ���Աȣ����pH�� | pH=11 | ˵�����ù����У���һ������______���ѧʽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�γ�����ˮ���п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ٳ�ȡ�����������ƹ�����Ʒ8.0g����50mLˮ�����Һ������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ���ã� | ������ɫ���� | ˵�����ù����У�һ������______���ѧʽ���� |
| ���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС���pH��ֽ�ϣ������ɫ���Աȣ����pH | pH=11 | ˵�����ù����У���һ������______���ѧʽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�γ����п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ٳ�ȡ�����������ƹ�����Ʒ8.0g����50mLˮ�����Һ������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ���ã� | ������ɫ���� | ˵�����ù����У�һ������______���ѧʽ���� |
| ���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС���pH��ֽ�ϣ������ɫ���Աȣ����pH | pH=11 | ˵�����ù����У���һ������______���ѧʽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
| ����Һ | NaCl | Na2CO3 | BaCl2 |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ٳ�ȡ�����������ƹ�����Ʒ8.0g����50mLˮ�����Һ������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ���ã� | ������ɫ���� | ˵�����ù����У�һ������______���ѧʽ���� |
| ���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС���pH��ֽ�ϣ������ɫ���Աȣ����pH | pH=11 | ˵�����ù����У���һ������______���ѧʽ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com