
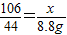 ����֮�ã�x=21.2g
����֮�ã�x=21.2g 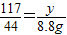 ����֮�ã�y=23.4g
����֮�ã�y=23.4g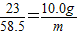 ��
��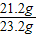 ×100%=91.4%
×100%=91.4%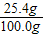 ×100%=25.4%
×100%=25.4%

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ϡ��������/g | 0 | 73 | 74 | 146 | 147 |
| �ձ�����Һ����/g | 200 | 273 | 273.76 | 328.4 | 329.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ���������������ѧ ���ͣ�������
ij�����������Ĵ����Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ�����������ij������ȤС�����������ʵ�顣ȡһ�������ĸô�����Ʒ���Թ��У�����85. 6gϡ���ᣬǡ����ȫ��Ӧ������8.8g���塣���ⶨ��������Һ������Ϊ��������Һ����Һ�к���Ԫ�ص�����Ϊ10. 0g������ݴ˷������㣺
(1)�ò�Ʒ��̼���Ƶ�����������____��
(2)��Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com