

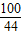 =
=
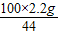 =5g
=5g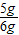 ×100%=83.3%
×100%=83.3%

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
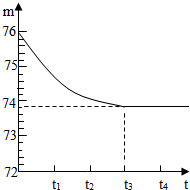 ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش�
ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
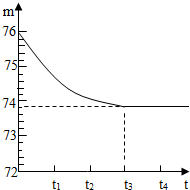 ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش�
ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
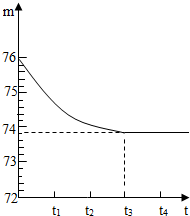 ��2011?������ģ�⣩����ɽλ��ʥ���������ϲ������зḻ��ʯ��ʯ�����Դ����ʯ��ʯ��Ʒ�к��ж����������ʣ�����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩��������ѧ��ͬѧ����ⶨ����Ʒ��̼��Ƶ��������������Dz�ȡ��һ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����50gһ��������������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ���������ʢ������������m���Ĺ�ϵ��ͼ��ʾ����ش�
��2011?������ģ�⣩����ɽλ��ʥ���������ϲ������зḻ��ʯ��ʯ�����Դ����ʯ��ʯ��Ʒ�к��ж����������ʣ�����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩��������ѧ��ͬѧ����ⶨ����Ʒ��̼��Ƶ��������������Dz�ȡ��һ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����50gһ��������������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ���������ʢ������������m���Ĺ�ϵ��ͼ��ʾ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ������к��������꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
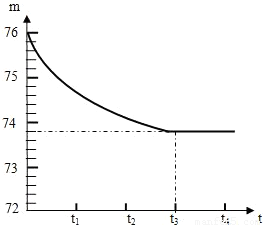 ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش�
ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����˽̰���꼶���ϣ���ѧ������⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
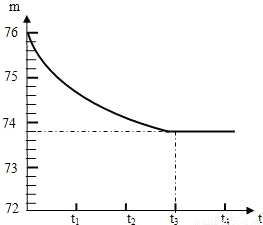 ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش�
ʯ��ʯ��Ʒ�к��ж����������ʣ���������Ȳ�����ˮҲ�������ᷴӦ����ijѧУ�Ļ�ѧ��ȤС���ͬѧ��ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����һ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ����������m���Ĺ�ϵ����ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com