
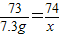
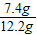 ×100%=60.7%
×100%=60.7%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
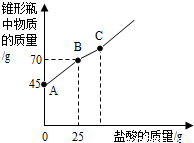
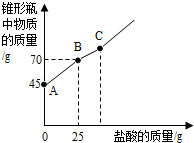 ��2012?üɽ���������Ƴ��ڴ�����ױ��ʣ�ij��ѧ̽��С����ʵ��ʱȡ��һƿ��Ŷ��������������������������������ȡ����������Ʒ12.2g����ƿ�У�����32.8gˮ��������γ�����Һ��Ȼ������ƿ����εμ�29.2%������ʹ���ַ�Ӧ��ʵ���ü����������������ƿ�����ʵ�������ϵ��ͼ��ʾ��
��2012?üɽ���������Ƴ��ڴ�����ױ��ʣ�ij��ѧ̽��С����ʵ��ʱȡ��һƿ��Ŷ��������������������������������ȡ����������Ʒ12.2g����ƿ�У�����32.8gˮ��������γ�����Һ��Ȼ������ƿ����εμ�29.2%������ʹ���ַ�Ӧ��ʵ���ü����������������ƿ�����ʵ�������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��Ĵ�üɽ������ѧ���������� ���ͣ�������
�������Ƴ��ڴ�����ױ��ʡ�ij��ѧ̽��С����ʵ��ʱȡ��һƿ��Ŷ��������������������������������ȡ����������Ʒ12.2g����ƿ�У�����32.8gˮ��������γ�����Һ��Ȼ������ƿ����εμ�29. 2%������ʹ���ַ�Ӧ��ʵ���ü����������������ƿ�����ʵ�������ϵ����ͼ��ʾ��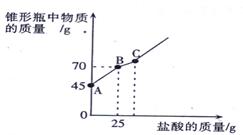
��(1)ͼ��AB�������ᷴӦ��������_ ___
(2)ͼ��BC�η�����Ӧ�Ļ�ѧ����ʽΪ
(3)��Ʒ���������Ƶ�������������ȷ��0.1��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
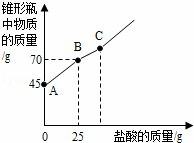
�������Ƴ��ڴ�����ױ��ʣ�ij��ѧ̽��С����ʵ��ʱȡ��һƿ��Ŷ��������������������������������ȡ����������Ʒ12.2g����ƿ�У�����32.8gˮ��������γ�����Һ��Ȼ������ƿ����εμ�29.2%������ʹ���ַ�Ӧ��ʵ���ü����������������ƿ�����ʵ�������ϵ��ͼ��ʾ��
��1��ͼ��AB�������ᷴӦ��������������
��2��ͼ��BC�η�����Ӧ�Ļ�ѧ����ʽΪ������
��3����Ʒ���������Ƶ�������������ȷ��0.1%��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
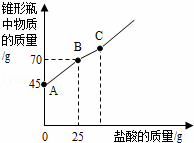
�������Ƴ��ڴ�����ױ��ʣ�ij��ѧ̽��С����ʵ��ʱȡ��һƿ��Ŷ��������������������������������ȡ����������Ʒ12.2g����ƿ�У�����32.8gˮ��������γ�����Һ��Ȼ������ƿ����εμ�29.2%������ʹ���ַ�Ӧ��ʵ���ü����������������ƿ�����ʵ�������ϵ��ͼ��ʾ��
��1��ͼ��AB�������ᷴӦ��������������
��2��ͼ��BC�η�����Ӧ�Ļ�ѧ����ʽΪ������
��3����Ʒ���������Ƶ�������������ȷ��0.1%��д��������̣���
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ�п����� ���ͣ�������
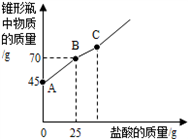
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com