

| 106 |
| x |
| 197 |
| 19.7g |
| 10.6g |
| 12.5g |
| 197 |
| 19.7g |
| 117 |
| y |
| 11.7g+12.5g-10.6g |
| 12.5g+107.2g-19.7g |


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
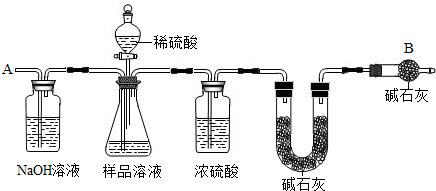
(13��)��֪ij������Ʒ�к��������Ȼ��ƣ�Ϊ�ⶨ��Ʒ�д���������������ס�������ֱ���ò�ͬ������
��������ͼװ��ͨ���ⶨ���������ټ�������������������
����Ҫ�������£�
�ٰ�ͼ��װ��������������ԣ�
�ڳ�ȡm������������ƿ�м���������ˮʹ֮��ȫ�ܽ⣻
�۳���װ��D��װ�м�ʯ�ҵ�U�ι�������a1�ˣ�
�ܴӷ�Һ©���������μ�20%��ϡ����
�ݴӵ���a�л������������
���ٴγ���װ��D��װ�м�ʯ�ҵ�U�ι�������
���ظ��ݺ͢IJ�����ֱ��װ��D��U�ι������������䣬�������Ϊa2�ˡ�
�Իش�������⣺
��Aװ�������װ______��Һ��������________________________��
��װ��C��Ũ�����������_____________________________________��
(3)������У��ӷ�Һ©���������μ�20%��ϡ����ֱ���۲쵽_______Ϊֹ��д���÷�Ӧ�Ļ�ѧ����ʽ______________________��
(4)��û�в���ݻ�ʹ�ⶨ���_______(ƫ���ƫС)��
(5)Eװ���м�ʯ�ҵ�������____________________________________________��
(6)װ��B�з�Һ©���ڵ�ϡ����ܻ���Ũ�����������__________________________��
�����������ɳ����ķ������ⶨ����(Na2CO3)��������������ȡ12.5����Ʒ����107.2�˵��Ȼ�����Һǡ����ȫ��Ӧ���˵õ��ij��������Ϊ19.7�˺�һ������Һ(��û����ʧ)���Լ������Ʒ�д������������������Һ�����ʵ�����������(д����5��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�������Ĵνο��Ի�ѧ�Ծ� ���ͣ�̽����
(13��)��֪ij������Ʒ�к��������Ȼ��ƣ�Ϊ�ⶨ��Ʒ�д���������������ס�������ֱ���ò�ͬ������
��������ͼװ��ͨ���ⶨ���������ټ�������������������

����Ҫ�������£�
�ٰ�ͼ��װ��������������ԣ�
�ڳ�ȡm������������ƿ�м���������ˮʹ֮��ȫ�ܽ⣻
�۳���װ��D��װ�м�ʯ�ҵ�U�ι�������a1�ˣ�
�ܴӷ�Һ©���������μ�20%��ϡ����
�ݴӵ���a�л������������
���ٴγ���װ��D��װ�м�ʯ�ҵ�U�ι�������
���ظ��ݺ͢IJ�����ֱ��װ��D��U�ι������������䣬�������Ϊa2�ˡ�
�Իش�������⣺
��Aװ�������װ______��Һ��������________________________��
��װ��C��Ũ�����������_____________________________________��
(3)������У��ӷ�Һ©���������μ�20%��ϡ����ֱ���۲쵽_______Ϊֹ��д���÷�Ӧ�Ļ�ѧ����ʽ______________________��
(4)��û�в���ݻ�ʹ�ⶨ���_______(ƫ���ƫС)��
(5)Eװ���м�ʯ�ҵ�������____________________________________________��
(6)װ��B�з�Һ©���ڵ�ϡ����ܻ���Ũ�����������__________________________��
�����������ɳ����ķ������ⶨ����(Na2CO3)��������������ȡ12.5����Ʒ����107.2�˵��Ȼ�����Һǡ����ȫ��Ӧ���˵õ��ij��������Ϊ19.7�˺�һ������Һ(��û����ʧ)���Լ������Ʒ�д������������������Һ�����ʵ�����������(д����5��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ����ϴ�����������ŭ�����ٲȾ�����ѧ ���ͣ������
(6��)��֪ij������Ʒ�к���NaCl���ʣ�Ϊ�ⶨ��Ʒ�д��������������������ͼ�е�װ�ý���ʵ��(��ܰ��ʾ����ʯ�ҵ���Ҫ�ɷ���NaOH��CaO)����Ҫʵ�鲽�����£�

�ٰ�ͼ��װ�����������װ�õ�������
�ڽ�ag��Ʒ������ƿ�У�����������ˮ�ܽ⣬�õ���Ʒ��Һ
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�bg
�ܴӷ�Һ©������ϡ���ᵽ���ٲ�������Ϊֹ
�ݴӵ���A����������һ�����Ŀ���
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�cg
���ظ�����ݺ͢IJ�����ֱ��ʢ�м�ʯ�ҵ�U�ܵ������������䣬Ϊdg
��ش��������⣺
(1)����������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵�� ��
(2)װ����NaOH��Һ�������� ��
(3)װ����Ũ����������� ��
(4)�������Һ©���е�ϡ���ỻ��Ũ����ͬ�����ᣬ�ⶨ�Ľ���� (��ƫ�ߡ�ƫ�ͻ�)��
(5)����ݵ�Ŀ���� ��
(6)����Ʒ�д�������������ļ���ʽΪ��_________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com