
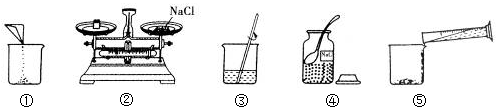
·ÖĪö £Ø1£©øł¾ŻŹµŃé²Ł×÷Ź¾ŅāĶ¼ÖŠĖłÉę¼°µÄ²£Į§ŅĒĘ÷”¢²£Į§°ōµÄ×÷ÓĆ£¬½ųŠŠ·ÖĪö½ā“š£®
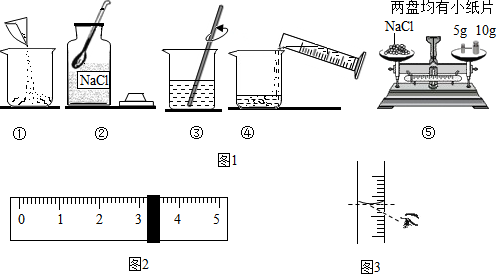
£Ø2£©øł¾ŻĶŠÅĢĢģĘ½µÄŹ¹ÓĆŅŖ×ńŃ”°×óĪļÓŅĀė”±µÄŌŌņ£¬½ųŠŠ·ÖĪö½ā“š£®
£Ø3£©ĄūÓĆČÜÖŹÖŹĮæ=ČÜŅŗÖŹĮæ”ĮČÜÖŹµÄÖŹĮæ·ÖŹż£¬æÉøł¾ŻČÜŅŗµÄÖŹĮæŗĶČÜÖŹµÄÖŹĮæ·ÖŹż¼ĘĖćÅäÖĘČÜŅŗĖłŠčŅŖµÄČÜÖŹµÄÖŹĮ棻ŌŁøł¾ŻČܼĮÖŹĮæ=ČÜŅŗÖŹĮæ-ČÜÖŹÖŹĮ漓æÉĒóµĆĖ®µÄÖŹĮ棬½ų¶ųČ·¶ØĮæĶ²µÄĮæ³Ģ£®
£Ø4£©øł¾ŻÅäÖĘČÜÖŹÖŹĮæ·ÖŹżŅ»¶ØµÄČÜŅŗµÄ»ł±¾²½Öč£¬½ųŠŠ·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©Ķ¼ÖŠµÄ²£Į§ŅĒĘ÷·Ö±šŹĒ¹ćæŚĘ攢ĮæĶ²”¢ÉÕ±ŗĶ²£Į§°ō£®Čܽā²Ł×÷ÖŠŠčŅŖŹ¹ÓĆ²£Į§°ō£¬²£Į§°ōŌŚ“Ė²Ł×÷ÖŠµÄ×÷ÓĆŹĒ½Į°č£¬¼ÓæģČܽāĖŁĀŹ£®
ÅäÖĘ100gČÜÖŹÖŹĮæ·ÖŹżĪŖ5%µÄNaClČÜŅŗ£¬Ź×ĻČ¼ĘĖćÅäÖĘČÜŅŗĖłŠčNaClŗĶĖ®µÄÖŹĮ棬ŌŁ³ĘĮæĖłŠčµÄNaClŗĶĮæČ”Ė®£¬×īŗó½ųŠŠČܽā£»ŌŚÕāŠ©²Ł×÷ÖŠŠčŅŖµÄŅĒĘ÷£ŗĶŠÅĢĢģĘ½”¢Ņ©³×”¢ĮæĶ²”¢½ŗĶ·µĪ¹Ü”¢ÉÕ±ŗĶ²£Į§°ō³żĮĖÉĻŹöŅĒĘ÷Ķā£¬»¹ŠčÓƵ½µÄŅĒĘ÷ÓŠŅ©³×”¢½ŗĶ·µĪ¹Ü£®
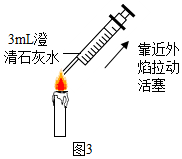
£Ø2£©ĶŠÅĢĢģĘ½µÄŹ¹ÓĆŅŖ×ńŃ”°×óĪļÓŅĀė”±µÄŌŌņ£¬Ķ¼ÖŠ³ĘĮæŹ±Ņ©Ę·ÓėķĄĀėµÄĪ»ÖĆ·Å·“ĮĖ£®
£Ø3£©ČÜÖŹÖŹĮæ=ČÜŅŗÖŹĮæ”ĮČÜÖŹµÄÖŹĮæ·ÖŹż£¬ÅäÖĘ100gČÜÖŹÖŹĮæ·ÖŹżĪŖ5%µÄNaClČÜŅŗ£¬ŠčĀČ»ÆÄʵÄÖŹĮæ=100g”Į5%=5g£»ČܼĮÖŹĮæ=ČÜŅŗÖŹĮæ-ČÜÖŹÖŹĮ棬ŌņĖłŠčĖ®µÄÖŹĮæ=100g-5g=95g£ØŗĻ95mL£©£»Ń”Č”ĮæĶ²Ź±£¬¾”ĮæŃ”ÓĆÄÜŅ»“ĪĮæČ”µÄ×īŠ”¹ęøńµÄĮæĶ²£®ÓĆĮæĶ²ĮæČ”95mLĖ®£¬Ó¦Ń”Ōń100mLµÄĮæĶ²£®
£Ø4£©ÅäÖĘ100gČÜÖŹÖŹĮæ·ÖŹżĪŖ5%µÄNaClČÜŅŗ£¬Ź×ĻČ¼ĘĖćÅäÖĘČÜŅŗĖłŠčNaClŗĶĖ®µÄÖŹĮ棬ŌŁ³ĘĮæĖłŠčµÄNaClŗĶĮæČ”Ė®£¬×īŗó½ųŠŠČܽā£¬¹ŹÅäÖĘČÜŅŗµÄ²Ł×÷Ė³ŠņŹĒ
¢Ü¢Ś¢Ł¢Ż¢Ū£®
¹Ź“š°øĪŖ£ŗ£Ø1£©ÉÕ±£»½Į°č£¬¼ÓæģČܽāĖŁĀŹ£»Ņ©³×”¢½ŗĶ·µĪ¹Ü£»£Ø2£©Ņ©Ę·ÓėķĄĀėµÄĪ»ÖĆ·Å·“ĮĖ£»£Ø3£©95£»100£»£Ø4£©¢Ü¢Ś¢Ł¢Ż¢Ū£®
µćĘĄ ±¾ĢāÄŃ¶Č²»“ó£¬Ć÷Č·Ņ»¶ØČÜÖŹÖŹĮæ·ÖŹżČÜŅŗµÄÅäÖĘµÄ²½Öč£Ø¼ĘĖć”¢³ĘĮ攢Čܽā£©”¢×¢ŅāŹĀĻīµČŹĒÕżČ·½ā“š“ĖĄąĢāµÄ¹Ų¼ü£®


 ×ß½ųĪÄŃŌĪÄĻµĮŠ“š°ø
×ß½ųĪÄŃŌĪÄĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
| ·½°øŅ» | ·½°ø¶ž£ØŹż×Ö»ÆŹµŃ飩 |
 |  |
| ·½°øŅ» | ·½°ø¶ž£ØŹż×Ö»ÆŹµŃ飩 |
 |  |
| ·½°øŅ» | ·½°ø¶ž£ØŹż×Ö»ÆŹµŃ飩 |
 |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | A2B3 | B£® | A2B5 | C£® | A3B4 | D£® | A4B3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
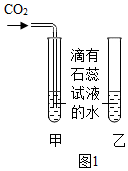
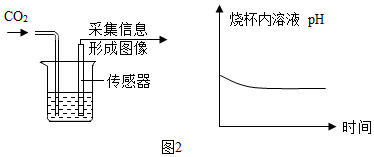
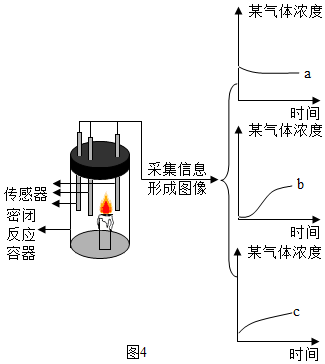
 ”°CO2ŅŖÓĆĻņÉĻÅÅæÕĘų·ØŹÕ¼Æ£¬²»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£®”±Š”æĘĶ¬Ń§ŅÉ»ó£ŗĪŖŹ²Ć“²»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£æÓŚŹĒ£¬½ųŠŠĮĖČēĻĀ¶Ō±ČŹµŃé£ŗÓĆĶ¼¼×ŗĶĶ¼ŅŅĮ½ÖÖ·½Ź½ŹÕ¼ÆCO2£¬ĶØČėµÄĘųĢåĮ÷ĖŁŗĶŹ±¼äĻąĶ¬£¬¾²ā¶Ø£¬·¢ĻÖ¼×¼ÆĘųĘæŹÕ¼Æµ½µÄCO2ÅØ¶Č½»“ó£¬ŅŅ¼ÆĘųĘæµÄCO2ÅØ¶Č½ĻŠ”£¬Ēė·ÖĪöĮ½ÕßÅضČĻą²ī½Ļ“óµÄŌŅņ£®
”°CO2ŅŖÓĆĻņÉĻÅÅæÕĘų·ØŹÕ¼Æ£¬²»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£®”±Š”æĘĶ¬Ń§ŅÉ»ó£ŗĪŖŹ²Ć“²»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£æÓŚŹĒ£¬½ųŠŠĮĖČēĻĀ¶Ō±ČŹµŃé£ŗÓĆĶ¼¼×ŗĶĶ¼ŅŅĮ½ÖÖ·½Ź½ŹÕ¼ÆCO2£¬ĶØČėµÄĘųĢåĮ÷ĖŁŗĶŹ±¼äĻąĶ¬£¬¾²ā¶Ø£¬·¢ĻÖ¼×¼ÆĘųĘæŹÕ¼Æµ½µÄCO2ÅØ¶Č½»“ó£¬ŅŅ¼ÆĘųĘæµÄCO2ÅØ¶Č½ĻŠ”£¬Ēė·ÖĪöĮ½ÕßÅضČĻą²ī½Ļ“óµÄŌŅņ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«Å©ŅµÉĻµÄ½ÕøĖ·ŪĖéŗó¾ĶµŲ»¹Ģļ | |
| B£® | µē³§”¢Ė®Äą³§Ō¶Ąė³ĒŹŠÅÅ·Åŗ¬PM2.5µÄŃĢ³¾ | |
| C£® | ÓŻƽØÖžÉč¼Ę£¬ŌöĒæŹŅÄŚ×ŌČ»²É¹ā£¬¼õÉŁÕÕĆ÷ÓƵē | |
| D£® | Ģį³«³Ė¹«½»»ņĘļ×ŌŠŠ³µ³öŠŠ£¬¼õÉŁĖ½¼Ņ³µµÄŹ¹ÓĆŹ±¼ä |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com