

·ÖĪö £Ø1£©øł¾Ż“ÖŃĪĢį“æµÄ²½ÖčæÉÖŖ£¬ŹĒÕō·¢£¬·ÖĪöø÷²½ĖłÓƵÄŅĒĘ÷£¬²¢ŅĄ¾ŻÕō·¢¹ż³ĢÖŠ²£Į§°ōµÄ×÷ÓĆ·ÖĪö½ā“š£®
£Ø2£©øł¾Ż¾«ŃĪÖʵĆĀŹ=¾«ŃĪÖʵĆĀŹ=$\frac{¾«ŃĪµÄÖŹĮæ}{“ÖŃĪµÄÖŹĮæ}$”Į100%·ÖĪö½ā“š£»
£Ø3£©øł¾ŻČÜŅŗČÜÖŹÖŹĮæ·ÖŹż=$\frac{ČÜÖŹÖŹĮæ}{ČÜŅŗÖŹĮæ}$”Į100%·ÖĪöÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©£©“ÖŃĪĢį“æµÄ²Ł×÷²½ÖčŹĒ£ŗČܽā”¢¹żĀĖ”¢Õō·¢”¢¼ĘĖć²śĀŹ£»ĘäÖŠŹ¹ÓĆʵĀŹ×ī¶ąµÄŅ»ÖÖ²£Į§ŅĒĘ÷ŹĒ²£Į§°ō£¬ŌŚÉĻŹöĮ÷³Ģ¢ŻÖŠÕō·¢Ź±ĪŖ·ĄÖ¹¾Ö²æ¹żČČ Ź¹ŅŗµĪ·É½¦£¬ŅŖÓĆ²£Į§°ō²»¶Ļ½Į°čµÄ·½·Ø£»
£Ø2£©¾«ŃĪÖʵĆĀŹ=$\frac{¾«ŃĪµÄÖŹĮæ}{“ÖŃĪµÄÖŹĮæ}$”Į100%¹ŹÕō·¢Ź±Ź³ŃĪ·É½¦¾ēĮŅ£¬²æ·ÖŹ³ŃĪ½¦³ö£¬¾«ŃĪÖŹĮæ¼õÉŁ£¬Ōņ¾«ŃĪ²śĀŹĘ«µĶ£»
£Ø3£©A”¢ĖłµĆµÄ¾«ŃĪ²»“棬ĖłµĆČÜŅŗÖŠŹ³ŃĪµÄÖŹĮ漓ČÜÖŹÖŹĮæĘ«Š”£¬¹ŹĖłÅäÖʵÄČÜŅŗČÜÖŹÖŹĮæ·ÖŹżĘ«Š”£»
B”¢ÅäÖĘČÜŅŗµÄÉÕ±ÓĆĖ®ČóĻ“Ć»øÉ£¬ĖłµĆČÜŅŗÖŠČܼĮĖ®µÄĮæĘ«“󣬹ŹĖłÅäÖʵÄČÜŅŗČÜÖŹÖŹĮæ·ÖŹżĘ«Š”£»
C”¢ÓĆĮæĶ²ĮæĖ®Ź±ŃöŹÓ¶ĮŹżČ”ÓƵÄĖ®µÄĮæĘ«Š”£¬ĖłÅäÖʵÄČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹżĘ«“ó£»
D”¢ĢģĘ½³ĘĮæ×óĪļÓŅĀė£¬²Ł×÷ÕżČ·£¬ÄÜ×¼Č·ÅäÖĘ³öĖłŠčµÄČÜŅŗ£»
¹Ź“š°øĪŖ£ŗ£Ø1£©Õō·¢ ²£Į§°ō ·ĄÖ¹¾Ö²æ¹żČČ Ź¹ŅŗµĪ·É½¦ £Ø2£©Ę«µĶ £Ø3£©ABC
µćĘĄ ±¾Ģāæ¼²éĮĖ“ÖŃĪµÄĢį“攢ČÜŅŗµÄÅäÖĘĻą¹ŲÖŖŹ¶£¬²ąÖŲ»ł“”ÖŖŹ¶µÄĮé»īÓ¦ÓĆ£®


 ½ĢѧĮ·ŠĀĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
½ĢѧĮ·ŠĀĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
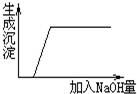
| A£® | HClŗĶBa£ØNO3£©2 | B£® | CuSO4ŗĶH2SO4 | C£® | CuSO4ŗĶNa2SO4 | D£® | Na2SO4ŗĶBa£ØNO3£©2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
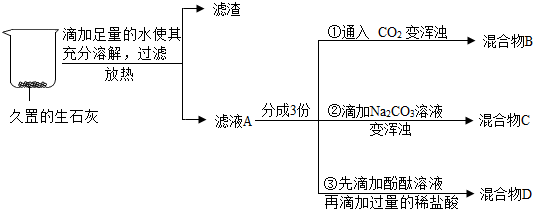
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆČ¼ÉÕľĢæµÄ·½·ØÖʱø“æ¾»µÄ¶žŃõ»ÆĢ¼ | |
| B£® | ÓĆŹŹĮæĻ”ĮņĖįĒå³żĢśÖĘĘ·±ķĆęµÄĢśŠā | |
| C£® | ÓĆ×ĻÉ«µÄŹÆČļČÜŅŗĒų·ÖŅ»Ńõ»ÆĢ¼ŗĶ¶žŃõ»ÆĢ¼ | |
| D£® | ÓĆ×ćĮæĻ”ŃĪĖį¼ģŃé¾ĆÖƵÄĒāŃõ»ÆÄĘ¹ĢĢåŹĒ·ń±äÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
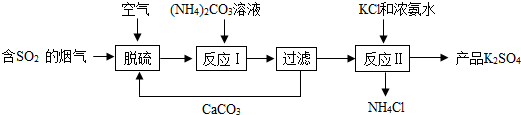
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
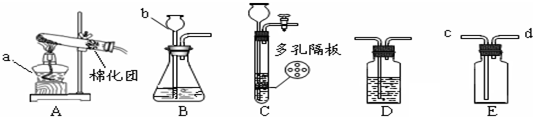
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
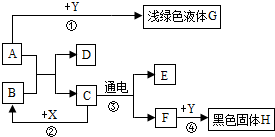 ČēĶ¼ĖłŹ¾£¬A”«HŹĒ³õÖŠ»Æѧ³£¼ūµÄĪļÖŹ£¬AŗĶBæÉ·¢ÉśÖŠŗĶ·“Ó¦£¬CĪŖÉś»īÖŠ×ī³£¼ūµÄŅŗĢ壬Fæɹ©øųŗōĪü£¬X³£ÓĆ×÷Ź³Ę·øÉŌļ¼Į£¬YĪŖµ„ÖŹ£¬Ēėøł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ĖłŹ¾£¬A”«HŹĒ³õÖŠ»Æѧ³£¼ūµÄĪļÖŹ£¬AŗĶBæÉ·¢ÉśÖŠŗĶ·“Ó¦£¬CĪŖÉś»īÖŠ×ī³£¼ūµÄŅŗĢ壬Fæɹ©øųŗōĪü£¬X³£ÓĆ×÷Ź³Ę·øÉŌļ¼Į£¬YĪŖµ„ÖŹ£¬Ēėøł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com