

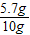 ×100%=57%���ʴ�Ϊ��57%��
×100%=57%���ʴ�Ϊ��57%�� ��
�� ��
�� ×100%=75%��
×100%=75%��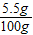 ×100%=5.5%��
×100%=5.5%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ѧ�����ǵ��������������ŷdz����е���ϵ�����ü�ͥ�����г�������ƷҲ�ܽ��кܶѧ̽�����������С�����ü��������Ʒ���е�ʵ��̽����
��1��С����ʯ����Һ�ֱ����״ʹ�����Һ�У�����ʯ���ڰ״��б��ɫ���ڴ�����Һ�б���ɫ��˵���״������ԣ�������Һ��pH__________7������ڡ�����С�ڡ����ڡ�����
��2���״���Ҫ�ɷ��Ǵ��ᣬ���кܶ����Ƶ����ʣ��磺������ʹʯ���� ����������ý�����Ӧ�����κ����� ��������Ӧ�����κ�ˮ ����������������ﷴӦ�����κ�ˮ ��������̼���η�Ӧ�����Ρ�ˮ�Ͷ�����̼�ȡ�Ϊʲô��ͬ���ᶼ�������ƻ�ѧ���ʣ�
С�����������������С�������У�����״ף�����_______ ��˵���״��������ⷴӦ��С���뵽Ŀǰ����������֯�����ƹ�ʹ���й����������ǽ�����ʹ����������ʱ�ŵ�ף����Բ���������Ҫ����Ԫ����Ԫ�أ�ԭ����__________������ţ���ͬʱ����__________������ţ���������Ϊ�����ð״�����ȥˮ���е�ˮ����
С�����״μӵ�������Һ�У�������Һ�������ݲ���������Ϊ�����кͷ�Ӧʱ����������̬��ˮ�����ʣ�С������ʶ��ȷ��Ϊʲô��
��3��С������ijʳƷ��װ��װ��һС������ʯ�Ҹ����������Ҫ�ɷ��������ƣ������˸��������ˮ�У�����ˮ���¶������ˣ�������Ϊ_____________________________________��
��4��С�������IJ��ϵ�֪������Ļ�ѧʽΪCH3COOH���������̼Ԫ�ص���������Ϊ_______________��
��5�������ǵ���Ҫ�ɷ���̼��ƣ�С����12.5g�����Ƿ���100g������ʳ���У���ַ�Ӧ��ʣ�����ʵ�����Ϊ108.1g�������ü������к���̼��Ƶ����������Ƕ��٣�����ʾ��2CH3COOH��CaCO3��Ca(CH3COO)2��H2O��CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ�����·��ѧ���꼶���£��¿���ѧ�Ծ���2�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com