
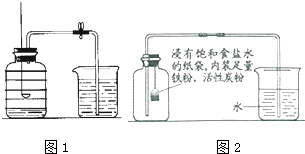
| ���� ��Ŀ | ʵ��ǰ | ʵ��� | |
| �ձ���ˮ����� | �ձ���ʣ��ˮ����� | ����ƿ���۳�������͵��ܵ��ݻ� | |
| ���/mL | 80.0 | 54.5 | 126.0 |
���� �������еĿ��������������IJⶨԭ�����з��������ȼ���������������ף����屻���ģ�ѹǿ��С���ʻ����ˮ�������������������֪ʶ���з�����ɣ�$\frac{25.5mL}{126mL}$
��� �⣺��1����ʣ����ײ���ȼ��֪������֧��ȼ�գ�
��2�����ܵ�ԭ���к��ײ��㡢װ��©����δ��װ����ȴ��
��3������̽�ֵ����������������Ӧ�������ˮ���ԣ����C��
ʵ��Ľ�����4������������ˮ��Ӧ��������������[Fe��OH��2]�����2Fe+2H2O+O2=2Fe��OH��2��
��5�����ݷ�Ӧǰ���ձ���ˮ������仯���Կ��������ĵ�����������ǣ�80-54.5=25.5mL�����������������Ϊ��$\frac{25.5mL}{126mL}$��100%��20.2%
��6��ʹ�����Ļ�����������������ʹ�������ĵĸ�Ϊ���ף�ʵ������ȷ������ʱ���ǵ����ݻ��Ϳ۳��������ļ���ƿ�������ʹʵ������Ϊȷ��������Ļ�������ʹ����ƿ�е��������ĸ�Ϊ���ף�ʹʵ������ȷ������ʱ���ǵ������ݻ��Ϳ۳��������ļ���ƿ�ݻ���ʹʵ������ȷ��
�ʴ�Ϊ����1����֧��ȼ�գ���2�����ײ��㡢װ��©������3��C����4��2Fe+2H2O+O2=2Fe��OH��2����5��20.2%����6�����Ļ�������ʹ����ƿ�е��������ĸ�Ϊ���ף�ʹʵ������ȷ��
���� ���⿼����������ĺ����IJⶨ�Լ�ʵ��װ�õĸĽ�����ɴ��⣬�����������е�֪ʶ���У�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����δ֪ | |
| �ڻ�ѧ����ʽ | |
| ��������� | |
| ����� | |
| ������ | |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ�������㣬����Ϊ���в�����Ԫ�� | |
| B�� | ͨ����ˮ������ˮ | |
| C�� | ˮ�����������ˮ���������ʲ��� | |
| D�� | ˮ������ԭ�ӹ��ɵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ȼ�� | B�� | ��ɫ���ܶ� | ||
| C�� | �ܽ��ԡ����� | D�� | ����������ũ�ҷʸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
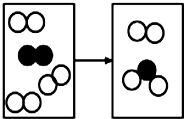 ��ͼ���ܱ���ϵ��ij��Ӧ����ʾ��ͼ�����𡱺͡��ֱ��ʾ��ͬԭ��
��ͼ���ܱ���ϵ��ij��Ӧ����ʾ��ͼ�����𡱺͡��ֱ��ʾ��ͬԭ�� B��
B�� C��
C�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
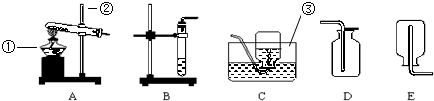
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com