



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Čż¾ŪĒč°·£Ø»ÆѧŹĒĪŖC3H6N6£©ÖŠĢ¼”¢Ēā”¢µŖČżŌŖĖŲµÄÖŹĮæ±ČĪŖ1£ŗ2£ŗ2 | B”¢Čż¾ŪĒč°·£Ø»ÆѧŹĒĪŖC3H6N6£©ÖŠµŖŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ66.7% | C”¢1øöČż¾ŪĒč°·£Ø»ÆѧŹĒĪŖC3H6N6£©·Ö×ÓÖŠŗ¬ÓŠ3øöN2·Ö×Ó | D”¢Čż¾ŪĒč°·£Ø»ÆѧŹĒĪŖC3H6N6£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖ126g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
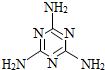 2008Äź9ŌĀ£¬ÖŠ¹ś±¬·¢ČżĀ¹Ó¤Ó׶łÄĢ·ŪŹÜĪŪČ¾ŹĀ¼ž£¬µ¼ÖĀŹ³ÓĆĮĖŹÜĪŪČ¾ÄĢ·ŪµÄÓ¤Ó׶ł²śÉśÉö½įŹÆ²”Ö¢£¬ĘäŌŅņŹĒÄĢ·ŪÖŠŗ¬ÓŠČż¾ŪĒč°·£Ø»ÆѧŹ½ĪŖC3N6H6£©£¬¹ŲÓŚČż¾ŪĒč°·ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
2008Äź9ŌĀ£¬ÖŠ¹ś±¬·¢ČżĀ¹Ó¤Ó׶łÄĢ·ŪŹÜĪŪČ¾ŹĀ¼ž£¬µ¼ÖĀŹ³ÓĆĮĖŹÜĪŪČ¾ÄĢ·ŪµÄÓ¤Ó׶ł²śÉśÉö½įŹÆ²”Ö¢£¬ĘäŌŅņŹĒÄĢ·ŪÖŠŗ¬ÓŠČż¾ŪĒč°·£Ø»ÆѧŹ½ĪŖC3N6H6£©£¬¹ŲÓŚČż¾ŪĒč°·ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A”¢Čż¾ŪĒč°·µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ126g | B”¢Čż¾ŪĒč°··Ö×ÓÖŠµŖŌŖĖŲµÄÖŹĮæ·ÖŹżŌ¼ĪŖ66.7% | C”¢Čż¾ŪĒč°·ÖŠĢ¼”¢µŖ”¢ĒāŌŖĖŲµÄÖŹĮæ±ČĪŖ3£ŗ6£ŗ6 | D”¢Čż¾ŪĒč°·ŹĒÓÉC”¢N”¢HČżÖÖŌŖĖŲ×é³ÉµÄ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
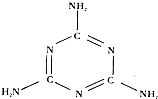 ŌŚŠĀµÄÅ£ÄĢ ¹ś¼Ņ±ź×¼ÖŠ£¬µ°°×ÖŹµÄŗ¬Įæ“ÓĆæ100æĖ²»µĶÓŚ 2.95%½µµĶµ½2.8%£®Čż¾ŪĒč°·ŹĀ¼žÖ®ĖłŅŌ±¬·¢£¬ÕżŹĒŅņĪŖ¹żČ„µÄÅ£ÄĢ±ź×¼Ēæµ÷µ°°×ÖŹŗ¬ĮæµÄŅŖĒóĢ«øßĮĖ£¬²æ·ÖÅ©»§µÄÅ£ÄĢ“ļ²»µ½ŅŖĒ󣬲Ų»Ļ§īś¶ų×ßĻÕ£®Čż¾ŪĒč°·µÄ»ÆѧŹ½ĪŖC3H6N6£Ø½į¹¹¼ūĶ¼£©£¬ŹĒŅ»ÖÖÓĆĶ¾¹ć·ŗµÄ»Æ¹¤ŌĮĻ£®øĆĪļÖŹĪŖ“æ°×É«¾§Ģ壬ĪŽĪ¶”¢ČÜÓŚČČĖ®£¬Ī¢ČÜÓŚĄäĖ®£®Ė®ČÜŅŗ³ŹČõ¼īŠŌ£®Ņ»°ćĒéæöĻĀ½ĻĪČ¶Ø£¬µ«ŌŚøßĪĀĻĀÄÜ·Ö½ā·Å³öÓŠ¶¾µÄĒč»ÆĪļ£®
ŌŚŠĀµÄÅ£ÄĢ ¹ś¼Ņ±ź×¼ÖŠ£¬µ°°×ÖŹµÄŗ¬Įæ“ÓĆæ100æĖ²»µĶÓŚ 2.95%½µµĶµ½2.8%£®Čż¾ŪĒč°·ŹĀ¼žÖ®ĖłŅŌ±¬·¢£¬ÕżŹĒŅņĪŖ¹żČ„µÄÅ£ÄĢ±ź×¼Ēæµ÷µ°°×ÖŹŗ¬ĮæµÄŅŖĒóĢ«øßĮĖ£¬²æ·ÖÅ©»§µÄÅ£ÄĢ“ļ²»µ½ŅŖĒ󣬲Ų»Ļ§īś¶ų×ßĻÕ£®Čż¾ŪĒč°·µÄ»ÆѧŹ½ĪŖC3H6N6£Ø½į¹¹¼ūĶ¼£©£¬ŹĒŅ»ÖÖÓĆĶ¾¹ć·ŗµÄ»Æ¹¤ŌĮĻ£®øĆĪļÖŹĪŖ“æ°×É«¾§Ģ壬ĪŽĪ¶”¢ČÜÓŚČČĖ®£¬Ī¢ČÜÓŚĄäĖ®£®Ė®ČÜŅŗ³ŹČõ¼īŠŌ£®Ņ»°ćĒéæöĻĀ½ĻĪČ¶Ø£¬µ«ŌŚøßĪĀĻĀÄÜ·Ö½ā·Å³öÓŠ¶¾µÄĒč»ÆĪļ£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com