
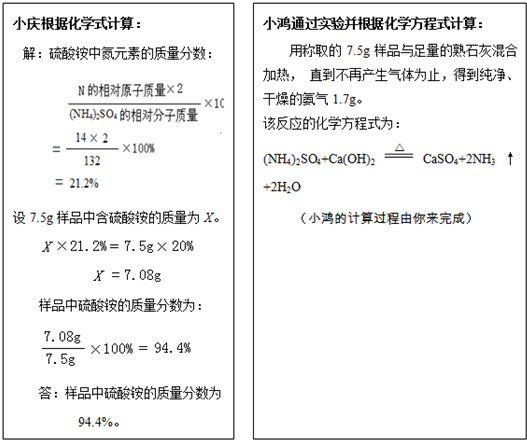
·ŹĢļ·ŪµÄÖ÷ŅŖ³É·ÖŹĒĮņĖįļ§”£ĻÖÓŠŅ»°ü±źÓŠŗ¬µŖĮæĪŖ20.0%µÄ·ŹĢļ·Ūѳʷ£¬Š”ĒģŗĶŠ”ŗčĪŖĮĖ¼ĘĖćøĆ·ŹĢļ·ŪÖŠĮņĖįļ§µÄÖŹĮæ·ÖŹż£¬ĖūĆĒ³ĘČ”7 .5gѳʷ£¬·Ö±š²ÉÓĆĻĀĮŠ·½·Ø£ŗ
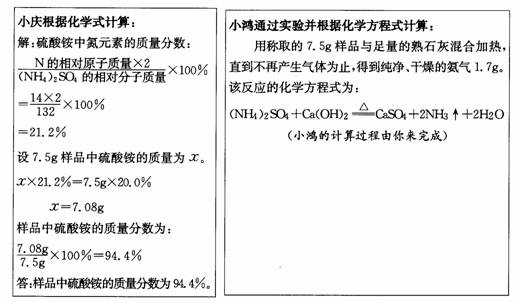
£Ø1£©ĒėÄćøł¾ŻŠ”ŗčµÄŹµŃé½į¹ū£¬¼ĘĖćѳʷ֊ĮņĖį°“µÄÖŹĮæ·ÖŹż”££Ø¼ĘĖć½į¹ū¾«Č·ÖĮ0 .1 % )
£Ø2£©ÄćµÄ¼ĘĖć½į¹ūŗĶŠ”ĒģµÄ¼ĘĖć½į¹ū__________ £ØĢī”°ĻąĶ¬”±»ņ”°²»ĻąĶ¬£¬' ) ,
ŌŅņŹĒ_______________________________________________ ”£
£Ø3£©øł¾ŻŠ”ŗ菵ŃéµÄ·“Ó¦ŌĄķ·ÖĪö£ŗŹ©ÓĆ·ŹĢļ·ŪŹ±Ó¦×¢Ņā___________________”£
£ØæÉÄÜÓƵ½µÄĻą¶ŌŌ×ÓÖŹĮæ£ŗH-1 C-12 N-14 O-16 S-32 £©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| NµÄĻą¶ŌŌ×ÓÖŹĮæ”Į2 |
| (NH2)2SO4µÄĻą¶Ō·Ö×ÓÖŹĮæ |
| 14”Į2 |
| 132 |
| 7.08g |
| 7.5g |
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2007Äźø£½ØŹ”ø£ÖŻŹŠÖŠæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com